- Title
-
Comparison of Pronase versus Manual Dechorionation of Zebrafish Embryos for Small Molecule Treatments
- Authors
- Hasegawa, E.H., Farr, G.H., Maves, L.
- Source
- Full text @ J Dev Biol
|
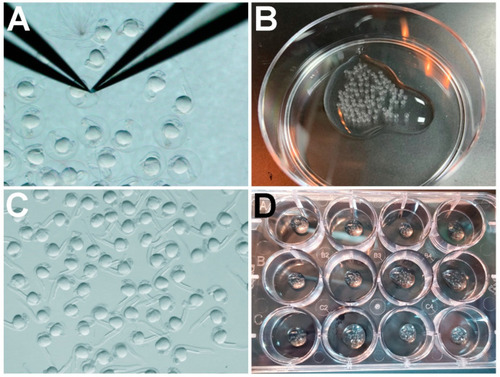
Zebrafish embryo dechorionation steps. (A) Using forceps to remove the chorions of 24 hpf embryos: (B) 24 hpf embryos transferred into a 60 mm × 15 mm dish prior to pronase treatment; (C) 24 hpf embryos immediately after 14 min treatment with 0.5 mg/mL pronase and 3X rinse with ICS water; (D) dechorionated 24 hpf embryos in a 12-well plate prior to treatment with TSA or control DMSO. |
|
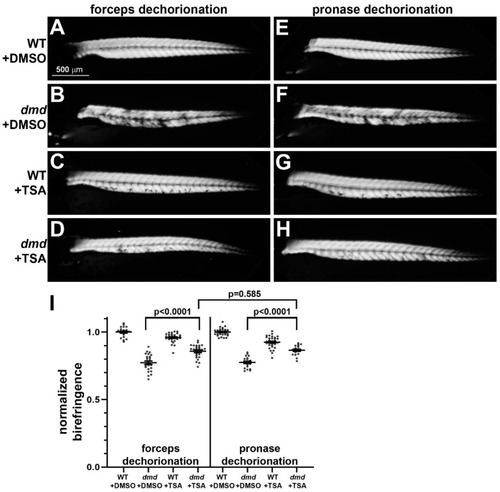
Muscle birefringence of TSA-treated zebrafish larvae following manual or pronase dechorionation. (A–H) Zebrafish trunk skeletal muscle birefringence at 4 dpf. Lateral views, anterior to the left. Images show (A,E) dmd+/+ wild-type (WT) + DMSO, (B,F) dmd+/+ WT + TSA, (C,G) dmd−/− (dmd) + DMSO, and (D,H) dmd−/− (dmd) + TSA. (I) Graph of the average normalized birefringence pixel intensities (mean grey values) in WT (dmd+/+) and dmd (dmd−/−) zebrafish larvae treated with DMSO (control), and dechorionated with forceps (left) or pronase (right). Horizontal lines represent the mean and standard error of the mean. Each dot represents an individual embryo (n = 18–31 animals per condition). Mean gray values between dmd + DMSO and dmd + TSA animals were significantly different for each dechorionation condition. The mean grey values between dmd + TSA animals were not significantly different when comparing each dechorionation condition. Error bars represent the standard error. Significance was determined using a one-way ANOVA test comparing each treatment group to the dmd DMSO control group with Dunnett’s correction for multiple comparisons. Data in support of (I) is available in Table S1 in Supplementary Materials. |
|
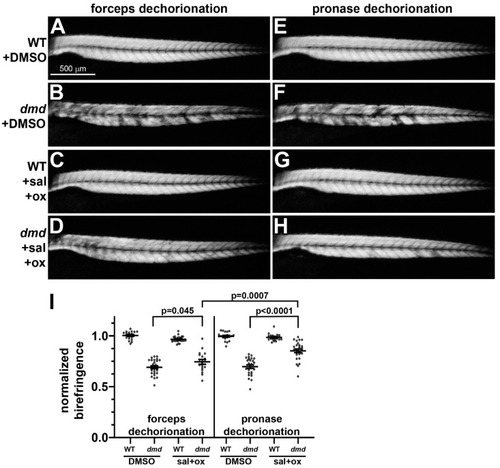
Muscle birefringence of salermide + oxamflatin-treated zebrafish larvae following manual or pronase dechorionation. (A–H) Zebrafish trunk skeletal muscle birefringence at 4 dpf. Lateral views, anterior to the left. Images show (A,E) dmd+/+ wild-type (WT) + DMSO, (B,F) dmd+/+ WT + salermide+oxamflatin, (C,G) dmd−/− (dmd) + DMSO, and (D,H) dmd−/− (dmd) + salermide+oxamflatin. (I) Graph of average normalized birefringence pixel intensities (mean grey values) in WT (dmd+/+) and dmd (dmd−/−) zebrafish larvae treated with DMSO (control) or salermide + oxamflatin and dechorionated with forceps (left) or pronase (right). Horizontal lines represent the mean and standard error of the mean. Each dot represents an individual embryo (n = 18–28 animals per condition). Mean gray values between dmd DMSO and dmd salermide + oxamflatin animals were significantly different for each dechorionation condition. Mean grey values for dmd salermide + oxamflatin pronase-dechorionated animals were significantly higher than those for dmd salermide + oxamflatin forceps-dechorionated animals. Error bars represent the standard error. Significance was determined using a one-way ANOVA test comparing each treatment group to the dmd DMSO control group with Dunnett’s correction for multiple comparisons. Data in support of (I) is available in Table S2 in Supplementary Materials. |
|
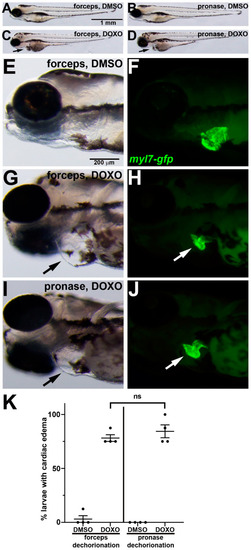
DOXO-induced cardiotoxicity in zebrafish embryos following manual or pronase dechorionation. (A–J) 96 hpf larvae that were treated from 24 hpf with (A,B,E,F) DMSO or (C,D,G–J) DOXO. Lateral views of larvae show anterior to the left. (E–J) Magnifications of head and cardiac region show paired brightfield and fluorescent images. Black arrows in (C,D,G,J) point to swelling around the heart. In (F,H,J), myl7-gfp (green) labels the myocardium, which is reduced in DOXO treatment ((H, |