- Title
-
Prolonged Piezo1 Activation Induces Cardiac Arrhythmia
- Authors
- Rolland, L., Torrente, A.G., Bourinet, E., Maskini, D., Drouard, A., Chevalier, P., Jopling, C., Faucherre, A.
- Source
- Full text @ Int. J. Mol. Sci.
|
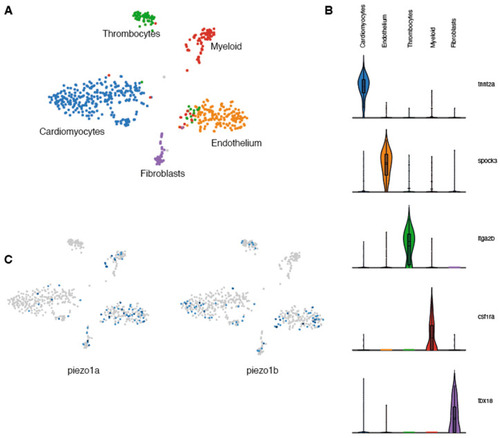
Single-nuclei RNAseq analysis of zebrafish Piezo expression in the heart. (A) UMAP clusters of the different populations of cells identified in zebrafish hearts. (B) Violin plots showing the 5 genes used to characterize the different cell populations. (C) UMAP plots indicating the cells expressing piezo1a or piezo1b. |
|
Electrophysiological recordings of zebrafish Piezos. (A–D) Cell-attached recordings obtained from HEK293T transfected with pIRES2-EGFP-piezo1a. n = 10 for each condition. (A) Representative zfPiezo1a traces obtained in untreated cells or cells treated with 30 µM Yoda1. (B) Current–pressure relationship. (C) I/Imax normalization fitted with Boltzmann equation. The average P50 was calculated from the fitting curve of each condition. (D) Proportion of inactivated channels 230 milliseconds after the beginning of a sweep (still under mechanical stimulation). (E) Area under curve calculated for cell-attached recordings. (F) Heterogeneity of the currents recorded in the cell-attached configuration in zf Piezo1b-transfected cells. (G) Representative whole-cell recordings obtained in cells transfected with either pIRES2-EGFP-piezo1a untreated (zf Piezo1a) or treated with 30 µM of Yoda1 (zf Piezo1a + 30 µM Yoda1) or pIRES2-EGFP-piezo1b (zf Piezo1b) and stimulated using mechanical indentations. (H) Representative deactivation current observed in the zf Piezo1a transfected cells treated with 30 µM of Yoda1. a,b: scale bars of zf Piezo1a (a) and zf Piezo1a + 30 µM Yoda1 (b), 1000 pA. (I) Proportion of current inactivated 200 milliseconds after the beginning of a sweep (still under mechanical stimulation). Data are presented as mean ± SEM. Statistical significance was assessed using two-way ANOVA followed by a Dunnett post hoc test for cell-attached recordings (B–E) and by t-test for the inactivation percentage of whole-cell recordings (I). *: p-value < 0.05, **: p-value < 0.01, ***: p-value < 0.001. |
|
Cardiac physiology of Yoda1-treated larvae. (A) Ventricular rate. (B) Atrial rate. (C) Ejection fraction. (D) QT interval. (E) Ventricular arrhythmia. (F) Atrial arrhythmia (G) End diastolic diameter. (H) Ventricle maximum fractional length. n = 5. Statistical significance was assessed by independent t-test and Bonferroni correction. *: p-value < 0.05, ****: p-value < 0.0001. |
|
ECG recordings of Yoda1-treated adult zebrafish. (A) Representative recordings obtained from the same fish before and after Yoda1 treatment. (B) Heart rate (in beats per minute, bpm) measured before and after a 1 h DMSO treatment; n = 5 (C) Heart rate measured before and after a 1 h Yoda1 treatment (50 µM); n = 7. (D) PR duration (in ms) measured before and after a 1 h DMSO treatment; n = 5 (E) PR duration measured before and after a 1 h Yoda1 treatment (50 µM); n = 6. Statistical significance was assessed by paired t-test. *: p-value < 0.05. |
|
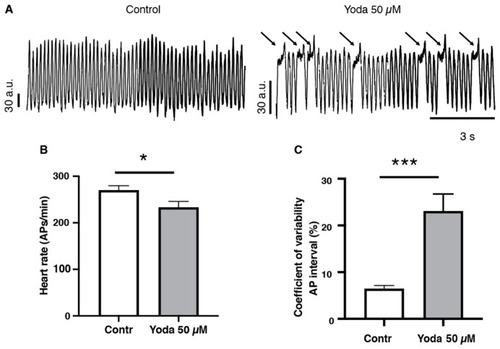
Optical mapping recordings of Yoda1-treated larvae. (A) Traces of optical action potentials recorded in the ventricular region of the larvae’s hearts, in control (DMSO) or after prolonged exposure to Yoda (50 µM). Arrows indicated prolonged ventricular depolarization. (B) Heart rate (ventricular action potentials per minute) and (C) coefficient of variability of the action potential interval in control (DMSO) and after prolonged exposure to Yoda 50 µM (n = 19 and 23, respectively). *: p < 0.05 and ***: p < 0.001 by unpaired t-test. |