- Title
-
Molecular Actors of Inflammation and Their Signaling Pathways: Mechanistic Insights from Zebrafish
- Authors
- Leiba, J., Özbilgiç, R., Hernández, L., Demou, M., Lutfalla, G., Yatime, L., Nguyen-Chi, M.
- Source
- Full text @ Biology (Basel)
|
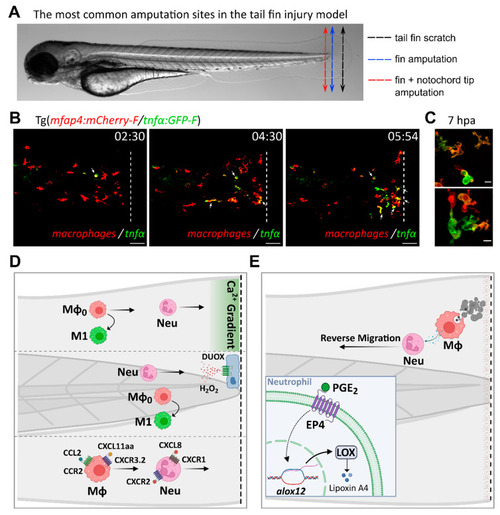
Inflammatory mediators and signaling pathways regulating leukocyte recruitment and activation during sterile injury, as inferred from the tail fin injury model. ( |
|
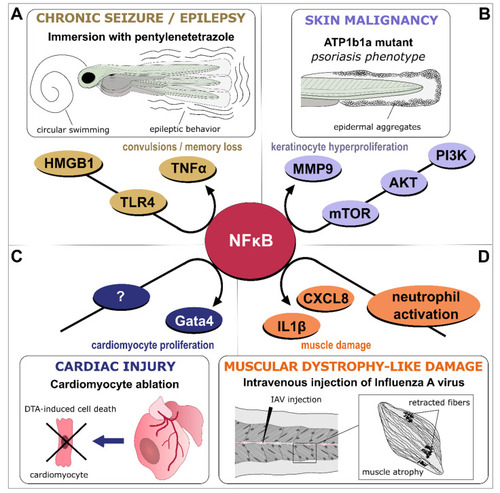
NFκB-dependent signaling as a central hub in inflammation. Various models of inflammatory-driven pathologies generated in larvae or adult zebrafish have identified NFκB signaling as part of the disease onset, progression, or recovery process. ( |
|
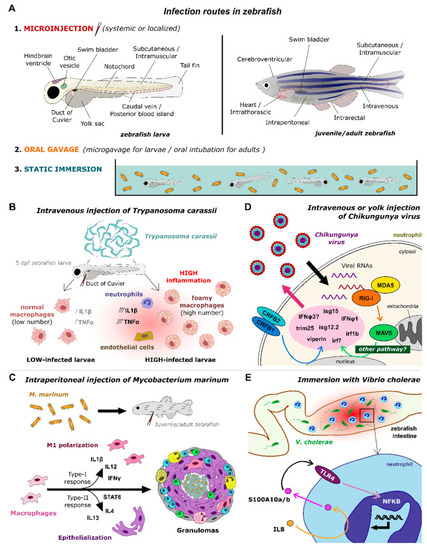
Signaling pathways unraveled by infection models in zebrafish. ( |