- Title
-
Regulatory factor identification for nodal genes in zebrafish by causal inference
- Authors
- Xing, C., Zeng, Z., Li, Y., Gong, B., Shen, W., Shah, R., Yan, L., Du, H., Meng, A.
- Source
- Full text @ Front Cell Dev Biol
|
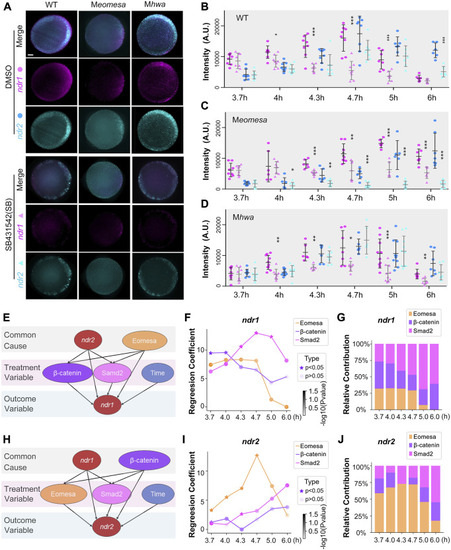
Regulatory factors of ndr1 and ndr2 analyzed by a causal graphical model. (A–D) Dynamic fluorescence intensities of ndr1 and ndr2 quantified for model analysis. (A) Representative expression pattern of ndr1 and ndr2 at 4.7 hpf detected by double fluorescence in situ hybridization (FISH). Embryos were treated with 1% DMSO or 50 μM SB431542 (SB) and fixed at indicated stages. All embryos are shown in animal-pole view with dorsal to the right; the dorsal is indicated by the expression pattern of ndr1/ndr2. It is of note that under SB treatment, the ndr1, not ndr2, could be detected in Meomesa embryos, while both ndr1 and ndr2 were present in Mhwa embryos. Scale bars, 100 μm. (B–D) Quantification of fluorescence intensities of ndr1 and ndr2 at indicated stages. In total, 7–10 embryos for each group were measured by Fiji for the total intensities. Pink dots, ndr1 intensities; blue dots, ndr2 intensities (circles for the DMSO group, and triangles for the SB group). It is of note that in Meomesa treated with DMSO, ndr2 intensities rise following the intensity increase of ndr1. Error bars represent s.d. for all embryos in each group. The asterisks show a significant difference between the SB group and the DMSO group. *p < 0.05; **p < 0.01; ***p < 0.001; not shown, not significant. (E–G) Hwa/β-catenin and Nodal/Smad2 could be the treatment variable factors for ndr1 expression during the time series. (E) Causal graphical model for ndr1 expression level. The causal inference was used to evaluate the causal between independent and dependent variables based on the linear regression model. The p-value of the regression coefficient of the independent variable (Eomesa) > 0.2 has no significant effect on the dependent variable ndr1, while the p-value of the regression coefficient of the independent variable (Hwa/β-catenin, Nodal/Smad2) < 0.2, indicating that these factors have a high contribution in the expression of ndr1. (F) The regression coefficient of Eomesa, β-catenin, and Smad2 on ndr1 at different time points. The p-value<0.5 was marked as a star. All coefficients were scaled down by a factor of 1000 for visualization. (G) The relative contribution of factors to ndr1 at different time points. (H–J) Eomesa and Smad2 could be the treatment variable factors for ndr2 expression during the time series. The data presentation was similar to those described in (E–G). |
|
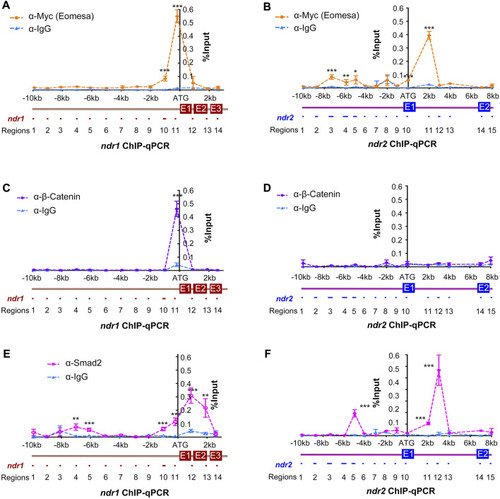
Identification of binding regions in ndr1 and ndr2 loci for Eomesa, β-catenin, and Smad2. Uninjected WT embryos or those injected with 50 pg Myc-eomesa mRNA were harvested at 4.3–5 hpf for chromatin immunoprecipitation using anti-Myc (A and B), anti-β-catenin (C and D), anti-Smad2 (E and F), or IgG antibody (Ab). The immunoprecipitated chromatin was used for quantitative PCR analysis using specific primers targeting different regions, as illustrated. The PCR results were normalized with the input genomic DNA with dilution factor (e.g., 1:10). The x-axis showed the genomic organization of ndr1 or ndr2 with amplified regions numbered. The translation start site was designated as position +1. The y-axis indicated the average percentage (±SEM) of amplified product relative to input DNA (% input) based on two independent experiments. Statistical significance levels: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, nonsignificant. |
|
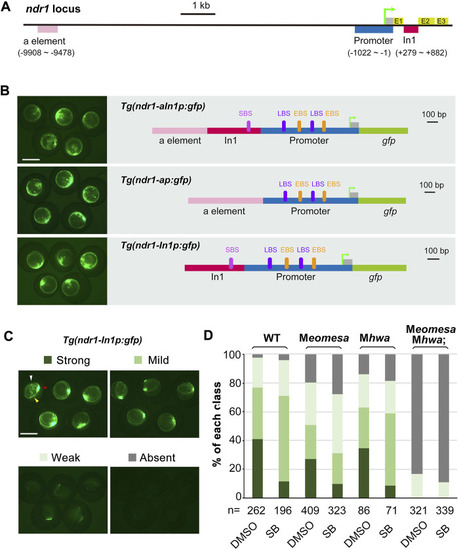
Regulation of ndr1 expression through transient transgenic assay. (A and B) Genomic organization of ndr1 locus (A) and composition of transgenes (B). The translation start site was designated as position +1. E, exons; In1, intron 1. EBS, Eomes-binding site; LBS, Lef/β-catenin binding site; SBS, Smad2-binding site. (C and D) Categorization of ndr1 transgenes in zebrafish embryos. Tg(ndr1-In1p:gfp) DNA was injected into one-cell stage WT or mutant embryos (50 pg/embryo), and GFP was observed at 4.3–5 hpf. For SB treatment, injected embryos were incubated in 50 μM SB or 1% DMSO (control) until observation. Based on GFP intensity in the blastodermal margin, embryos were categorized into four classes, as shown for WT embryos (top panel). The expression domain in the blastodermal margin is indicated by yellow heads, and the domain in the dorsal margin is indicated by red arrowheads in typical embryos. The presumably nonspecific signals are indicated by white arrowheads. Scale bars: 500 μm. The bar graphs (bottom) show the ratios of embryos in each class in WT or different mutants. n, the number of observed embryos. |
|
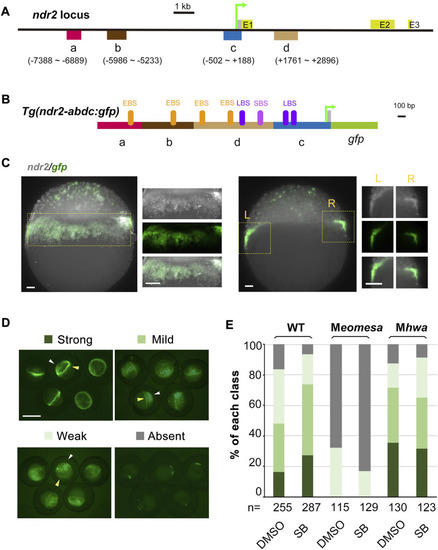
Verification of ndr2 cis-regulatory regions through transient transgenic assay. (A–C) Genomic organization of ndr2 locus (A) and composition of transgenes (B) with specific gfp expression mimicking endogenous ndr2 (C). The translation start site was designated as position +1. Exons (E) of ndr2 were indicated. EBS, Eomes-binding site; LBS, Lef/β-catenin binding site; SBS, Smad2-binding site. Wild-type embryos were injected with 50 pg Tg(ndr2-abdc:gfp) and harvested at about 30% epiboly stage for detection of ndr2 and gfp expression pattern by FISH. Lateral views (the left panel) and vertical sections (the right panel) with magnification (indicated in the yellow rectangle) were shown. Scale bars: 50 μm. (D–E) Categorization of ndr2 transgenes in zebrafish embryos. Tg(ndr2-abdc:gfp) DNA was injected into one-cell stage WT or mutant embryos (50 pg/embryo), and GFP was observed at 4.3–5 hpf. The embryo treatment and data presentation were similar to those described in Figure 3C and 3D. |