- Title
-
Function conservation and disparities of zebrafish and human LGP2 genes in fish and mammalian cells responsive to poly(I:C)
- Authors
- Gong, X.Y., Qu, Z.L., Li, Y.L., Sun, H.Y., Zhao, X., Dan, C., Gui, J.F., Zhang, Y.B.
- Source
- Full text @ Front Immunol
|
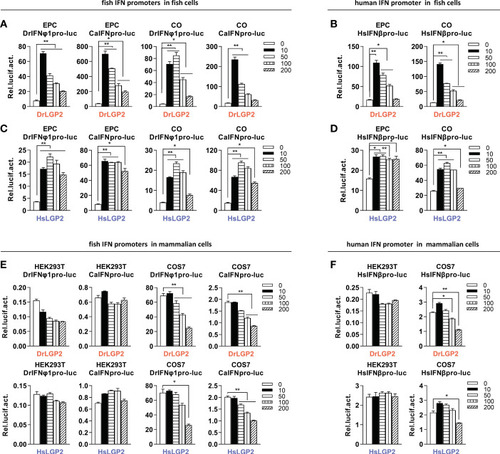
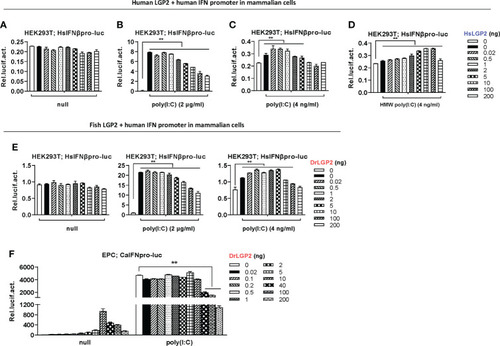
Overexpression of zebrafish or human LGP2 facilitates the activation of fish and human promoters in fish cells but not in mammalian cells (A–D) DrLGP2 and HsLGP2 both activated fish IFN promoters (A, C) and human IFNβ promoter (B, D) in fish cells. EPC cells and CO cells seeded overnight in 24-wells plates were co-transfected with DrIFNφ1pro-luc or CaIFNpro-luc (A, C), or HsIFNβpro-luc (200 ng) (B, D), together with DrLGP2 (A, B) or HsLGP2 (C, D) at increasing doses (0, 10, 50, 100, 200 ng). pRL-TK (20 ng) was transfected as internal control. 48 h later, cells were collected for luciferase assays. (E, F) DrLGP2 and HsLGP2 did not activate fish IFN promoters (E) and human IFNβ promoter (F) in mammalian cells. HEK293T cells and COS7 cells seeded overnight in 24-wells plates were co-transfected with DrIFNφ1pro-luc or CaIFNpro-luc (E), or HsIFNβpro-luc (200 ng) (F), together with DrLGP2 or HsLGP2 at increasing doses (0, 10, 50, 100, 200 ng). Renilla vector (pRL-TK, 0.2 ng) was transfected as internal control. 48 h later, cells were collected for luciferase assays. Error bars show the SDs of triplicate transfections. Data were shown as mean ± SD (N=3). P values were calculated using ANOVA. **P < 0.01, *P < 0.05. |
|
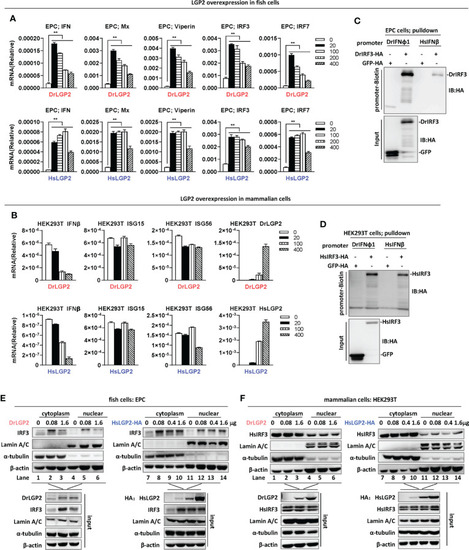
Overexpression of zebrafish or human LGP2 induces IFN response in fish cells but not in mammalian cells (A, B) RT-PCR analysis of transcriptional levels of IFN and ISGs induced by DrLGP2 and HsLGP2 in EPC cells (A) and HEK293T cells (B). EPC cells (A) and HEK293T cells (B) seeded overnight in 12-wells plates were transfected with DrLGP2 or HsLGP2 at increasing doses (0, 20, 10, 200, 400 ng) for 24 h, followed by RT-PCR detection of cellular gene transcription. P values were calculated using ANOVA. **P < 0.01. (C, D) DNA pull-down assays verified the binding of DrIFNφ1 and HsIFNβ promoter DNA to DrIRF3 in EPC cells (C) and HsIRF3 in HEK293T cells (D). EPC cells (C) and HEK293T cells (D) seeded in 10 cm dishes were transfected with DrIRF3-HA (C) or HsIRF3-HA (D). GFP-HA was transfected in parallel as control. 24 h later, cells were lysed. One-tenth of cell lysates were taken as input, and the remaining was incubated overnight with 100 ng biotinylated DrIFNφ1 promoter DNA (-596 to +38) (C) or HsIFNβ promoter DNA (-338 to +93) (D). The DNA-bound protein complexes were detected by western blots with anti-HA antibody. (E, F) overexpression of LGP2 increased nuclear IRF3 protein levels in fish cells but not in mammalian cells. EPC cells (E) and HEK293T cells (F) seeded in 5 cm dishes were transfected with DrLGP2 or HsLGP2 at increasing doses. 24 h later, cells were collected for nuclear and cytoplasmic separation, followed by western blot analyses of the indicated proteins using corresponding antibodies. The expression of Lamin A/C and α-tubulin verified the successful separation of nuclear and cytoplasmic lysates. |
|
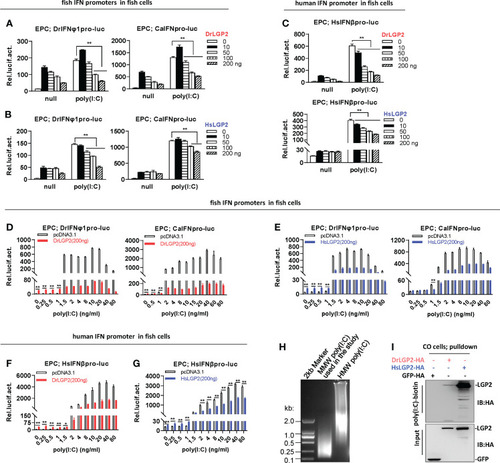
Zebrafish and human LGP2s play antithetic roles in regulating IFN response by poly(I:C) in fish cells (A–C) DrLGP2 and HsLGP2 downregulated fish IFN and human IFN promoter activation by poly(I:C) at a high concentration of 1 μg/ml in EPC cells. EPC cells seeded in 24-wells plates were co-transfected with DrIFNφ1pro-luc or CaIFNpro-luc (A, B), or HsIFNβpro-luc (200 ng each) (C), together with DrLGP2 or HsLGP2 at increasing doses (0, 10, 50, 100, 200 ng). 24 h later, cells were transfected with 1 μg/ml poly(I:C) for another 24 h, followed by luciferase assays. P values were calculated using ANOVA. **P < 0.01,*P < 0.05. (D–G) DrLGP2 and HsLGP2 switched a first positive role to a following negative one in regulating IFN response by increasing concentrations of poly(I:C) in EPC cells. EPC cells seeded overnight in 24-wells plates were co-transfected with DrIFNφ1pro-luc or CaIFNpro-luc (D, E), or HsIFNβpro-luc (F, G), together with DrLGP2 (D, F) or HsLGP2 (E, G) (200 ng each). 24 h later, cells were transfected with poly(I:C) at increasing doses for another 24 h, followed by luciferase assays. P values were calculated using Student’s t-test. **P<0.01. (H) Agarose electrophoresis showed the molecular weight spanning of MMW poly(I:C) and HMW poly(I:C). (I) RNA pull-down assays verified the binding of poly(I:C) to DrLGP2 and HsLGP2 in fish cells. CO cells seeded in 10 cm dishes were transfected with DrLGP2-HA, HsLGP2-HA or GFP-HA as control. 24 h later, cells were lysed. One-tenth of cell lysates were taken as input, the remaining was incubated with 100 ng biotinylated poly(I:C), followed by western blots with anti-HA antibody. |
|
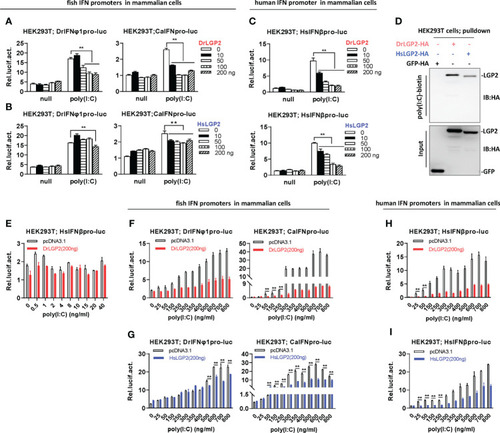
Zebrafish and human LGP2s play a negative role in regulating IFN response by high concentrations of poly(I:C) in mammalian cells (A–C) DrLGP2 and HsLGP2 downregulated fish IFN and human IFNβ promoter activation by poly(I:C) at a high concentration of 2 μg/ml in mammalian cells. HEK293T cells seeded in 24-wells plates were co-transfected with DrIFNφ1pro-luc or CaIFNpro-luc (A, B), or HsIFNβpro-luc (200 ng each) (C), together with DrLGP2 or HsLGP2 at increasing doses (0, 10, 50, 100, 200 ng). 24 h later, cells were transfected with 2 μg/ml poly(I:C) for another 24 h, followed by luciferase assays. P values were calculated using ANOVA. **P < 0.01. (D) RNA pull-down assays verified the binding of poly(I:C) to DrLGP2 and HsLGP2 in mammalian cells. HEK293T cells seeded in 10 cm dishes were transfected with DrLGP2-HA, HsLGP2-HA or GFP-HA as control. 24 h later, cells were lysed. One-tenth of cell lysates were taken as input, the remaining was incubated with 100 ng biotinylated poly(I:C), followed by western blots with anti-HA antibody. (E–I) Titration of poly(I:C) revealed a negative regulation of zebrafish and human LGP2s on IFN response in mammalian cells. HEK293T cells seeded in 24-wells plates were co-transfected with HsIFNβpro-luc (E, H, I), or with DrIFNφ1pro-luc or CaIFNpro-luc (F, G), together with DrLGP2 (E, F, H) or HsLGP2 (G, I) (200 ng each). Renilla vector (pRL-TK, 0.2 ng) was transfected as internal control. 24 h later, cells were transfected with poly(I:C) at increasing doses for another 24 h, followed by luciferase assays. P values were calculated using Student’s t-test. **P < 0.01. |
|
Zebrafish and human LGP2s play antithetic roles under low concentrations of poly(I:C) in mammalian cells and do so alone in fish cells. (A–E) Zebrafish and human LGP2s played antithetic roles under low concentrations of poly(I:C) in mammalian cells. HEK293T cells seeded in 24-wells plates were co-transfected with HsIFNβpro-luc (200ng), together with HsLGP2 (A–D) or DrLGP2 (E) (200 ng each). 24h later, cells were transfected again with MMW poly(I:C) [indicated as poly(I:C) in the text or all Figures] at 2 μg/ml (B, E) or at 4 ng/ml (C), or with HMW poly(I:C) at 4 ng/ml (D). Renilla vector (pRL-TK, 0.2 ng) was transfected as internal control. Another 24 h later, cells were collected for luciferase assays. P values were calculated using ANOVA. **P < 0.01. (F) Overexpression of zebrafish or human LGP2s alone revealed antithetic roles in mammalian cells. EPC cells seeded in 24-well plates were transfected with DrLGP2 at the indicated increasing doses for 48 h. Or at 24 h post transfection, cells were transfected again with poly(I:C) at a high concentration of 1 μg/ml for another 24 h, followed by luciferase assays. P values were calculated using ANOVA. **P < 0.01. |
|
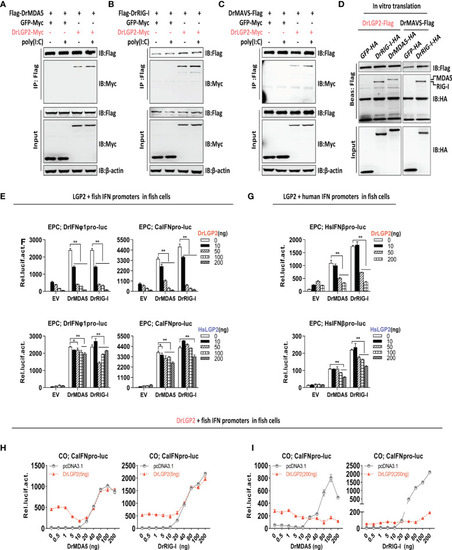
Zebrafish and human LGP2s play antithetic roles in regulating MDA5-/RIG-I-triggered IFN response in fish cells dependently of the exact doses of MDA5/RIG-I (A–C) Zebrafish LGP2 bound to DrMDA5 (A), DrRIG-I (B) or DrMAVS (C) independently of poly(I:C) by Co-IP assays. HEK293T cells seeded in 10 cm dishes overnight were transfected with LGP2-myc, together with Flag-DrMDA5 (A), Flag-DrRIG-I (B), DrMAVS-Flag (C) (5 μg each), in the presence or absence of poly(I:C) at 200 ng/ml) (A–C). Cell lysates were immunoprecipitated with anti-Flag Ab, followed by western blot analysis of the immunoprecipitates with anti-myc Ab. (D) In vitro-translated DrLGP2 bound to in vitro-translated DrMDA5 and DrRIG-I by pull-down assays. 50 μl of in vitro-translated DrLGP2-Flag was incubated with in vitro-translated DrMDA5-HA or in vitro-translated DrRIG-I-HA in NP40 lysis buffer at 4°C for 12 h. The bead-bound protein complex was performed by western blotting analysis with anti-Tag Ab. In vitro-translated DrMAVS-Flag was incubated with in vitro-translated DrRIG-I-HA as positive control, and with GFP-HA as negative control. (E–G) DrLGP2 and HsLGP2 downregulated IFN promoter activation in fish cells induced by DrMAD5 or DrRIG-I at a high dose. EPC cells seeded in 24-wells plates were transfected with DrIFNφ1pro-luc or CaIFNpro-luc (E, F) or HsIFNβpro-luc (G), DrMAD5 or DrRIG-I (200 ng each), DrLGP2 or HsLGP2 at increasing doses (0, 10, 50, 100, 200 ng) for 24 h, followed by luciferase assays. P values were calculated using ANOVA. **P<0.01, *P<0.05. (H, I) Titration of DrMDA5 and DrRIG-I revealed DrLGP2-mediated antithetic regulation of IFN response in fish cells. CO cells seeded in 24-well plates were transfected with CaIFNpro-luc (200 ng), DrLGP2 at 5 ng (H) or at 200 ng (I), DrMDA5 or DrRIG-I at increasing doses for 24 h, and finally collected for luciferase assays. |
|
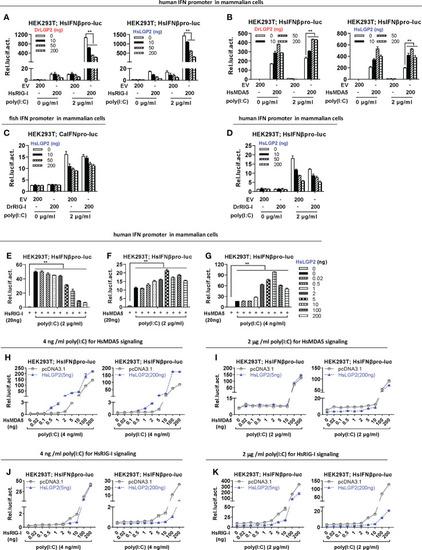
Zebrafish and human LGP2s promote MDA5 signaling under low concentrations of poly(I:C) and downregulate RIG-I/MDA5 signaling mainly under high concentrations of poly(I:C) in mammalian cells (A, B) DrLGP2 and HsLGP2 promoted human IFNβ activation by HsMDA5 at a high dose and downregulated IFN response by HsRIG-I at a high dose in mammalian cells. HEK293T cells seeded in 24-wells plates were transfected with HsIFNβpro-luc, DrLGP2 or HsLGP2 at increasing doses, together with HsRIG-I (A) or HsMAD5 (B) (200 ng each). 24 h later, cells were transfected again with or without poly(I:C) (2 μg/ml) for another 24 h, followed by luciferase assays. P values were calculated using ANOVA. **P<0.01. (C, D) HsLGP2 negatively regulated zebrafish RIG-I signaling in mammalian cells. HEK293T cells seeded in 24-wells plates were transfected as in A with the indicated plasmids. (E–G) Titration of HsLGP2 expression revealed differential regulation of RIG-I- and MDA5-triggered IFN signaling by HsLGP2 in the presence of poly(I:C) in mammalian cells. HEK293T cells seeded in 24-wells plates were transfected with HsIFNβpro-luc, HsLGP2 at increasing doses, together with HsRIG-I (E) or HsMAD5 (F, G) (200 ng each). 24 h later, cells were transfected with or without poly (I:C) at 2 μg/ml (E, F) or at 4 ng/ml (G) for another 24 h, followed by luciferase assays. P values were calculated using ANOVA. **P<0.01. (H–K) Titration of HsMDA5/MsRIG-I showed that HsLGP2 promoted HsMDA5 signaling under low concentrations of poly (I:C) and downregulate HsRIG-I signaling mainly under high concentrations of poly (I:C) in mammalian cells. HEK293T cells seeded in 24-wells plates were transfected with HsIFNβpro-luc, HsLGP2, together with increasing doses of HsMDA5 (H, K) or HsRIG-I (J, K). 24 h later, cells were transfected with or without poly (I:C) at 4 ng/ml (H, J) or 2 μg/ml (I K) for another 24 h, followed by luciferase assays. |