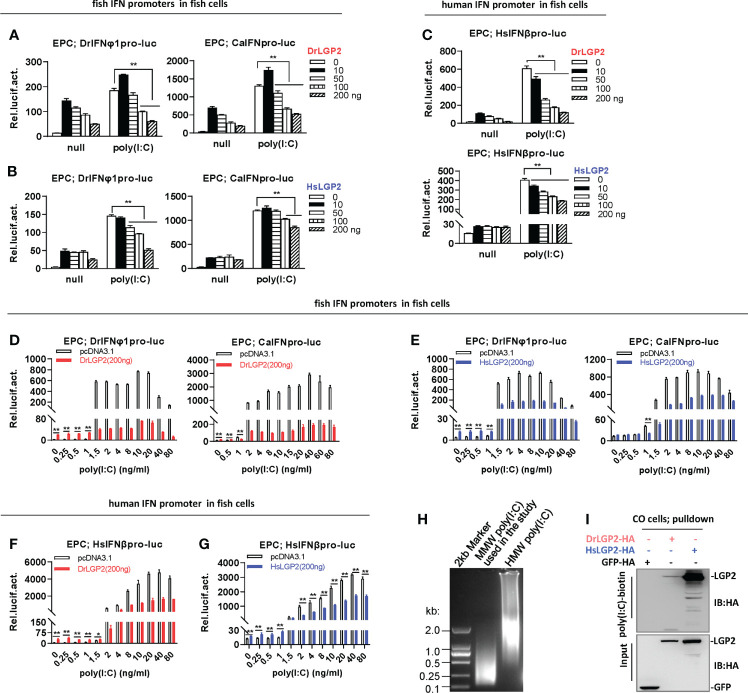
Fig. 3
Zebrafish and human LGP2s play antithetic roles in regulating IFN response by poly(I:C) in fish cells (A–C) DrLGP2 and HsLGP2 downregulated fish IFN and human IFN promoter activation by poly(I:C) at a high concentration of 1 μg/ml in EPC cells. EPC cells seeded in 24-wells plates were co-transfected with DrIFNφ1pro-luc or CaIFNpro-luc (A, B), or HsIFNβpro-luc (200 ng each) (C), together with DrLGP2 or HsLGP2 at increasing doses (0, 10, 50, 100, 200 ng). 24 h later, cells were transfected with 1 μg/ml poly(I:C) for another 24 h, followed by luciferase assays. P values were calculated using ANOVA. **P < 0.01,*P < 0.05. (D–G) DrLGP2 and HsLGP2 switched a first positive role to a following negative one in regulating IFN response by increasing concentrations of poly(I:C) in EPC cells. EPC cells seeded overnight in 24-wells plates were co-transfected with DrIFNφ1pro-luc or CaIFNpro-luc (D, E), or HsIFNβpro-luc (F, G), together with DrLGP2 (D, F) or HsLGP2 (E, G) (200 ng each). 24 h later, cells were transfected with poly(I:C) at increasing doses for another 24 h, followed by luciferase assays. P values were calculated using Student’s t-test. **P<0.01. (H) Agarose electrophoresis showed the molecular weight spanning of MMW poly(I:C) and HMW poly(I:C). (I) RNA pull-down assays verified the binding of poly(I:C) to DrLGP2 and HsLGP2 in fish cells. CO cells seeded in 10 cm dishes were transfected with DrLGP2-HA, HsLGP2-HA or GFP-HA as control. 24 h later, cells were lysed. One-tenth of cell lysates were taken as input, the remaining was incubated with 100 ng biotinylated poly(I:C), followed by western blots with anti-HA antibody.