- Title
-
The centriolar satellite protein Cfap53 facilitates formation of the zygotic microtubule organizing center in the zebrafish embryo
- Authors
- Willekers, S., Tessadori, F., van der Vaart, B., Henning, H.H., Stucchi, R., Altelaar, M., Roelen, B.A.J., Akhmanova, A., Bakkers, J.
- Source
- Full text @ Development
|
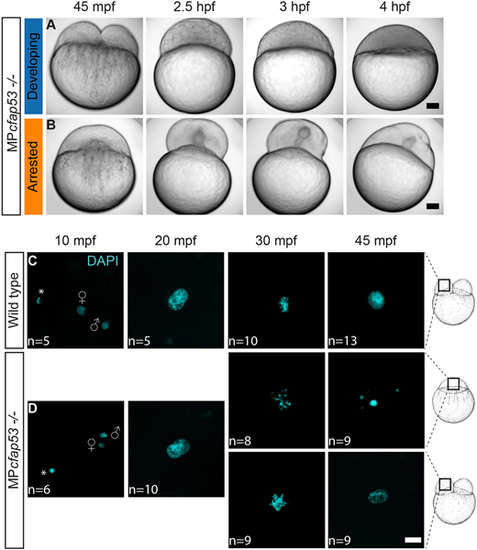
MPcfap53−/− embryos arrest and display aberrant nuclear morphology. (A,B) Time series of maternal and paternal (MP)cfap53−/− embryos from 45 mpf up to 4 hpf. Although a small fraction of the MPcfap53−/− embryos displayed normal development (A, n=15), most embryos arrested at the one-cell stage (B, n=52). (C,D) Confocal images of wild-type (C) and MPcfap53−/− (D) embryos at indicated stages of development stained with DAPI to visualize nuclei, indicating that aberrant nuclear morphology starts to form at 30 mpf. Results were obtained from two different cfap53−/− pairs. Asterisks indicate polar bodies. Gender symbols indicate a male or female pro-nucleus. Scale bars: 100 μm in A,B; 20 μm in C,D. Schematics of embryonic development are adapted, with permission, from Kimmel et al. (1995). |
|
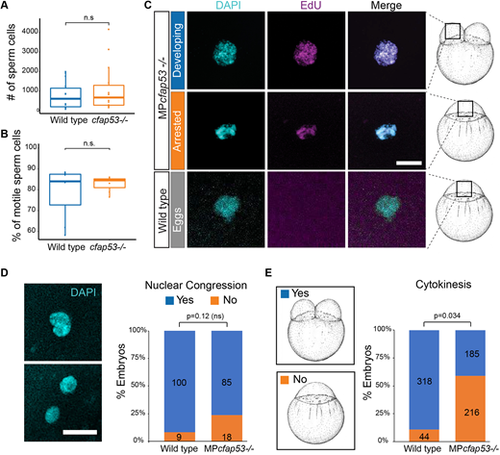
cfap53−/− fish are fertile. (A,B) Boxplots for sperm cell analysis showing the total number (horizontal lines) of sperm cells counted (A) and percentage of motile sperm cells (B) in spermatozoa derived from wild type (n=7) and cfap53−/− males (n=7). Boxes indicate upper and lower quartiles and whiskers indicate upper and lower extremes. (C) Confocal images showing DAPI staining and EdU incorporation in MPcfap53−/− embryos and lack of EdU incorporation in unfertilized eggs. EdU incubation was performed from 0 to 60 mpf. Quantification of the results are presented in Table 2. (D) Confocal images of one-cell stage embryos stained with DAPI showing either lack of nuclear congression (bottom) or initiation of nuclear congression (top) of male and female pronuclei. Graph shows a quantification of observed nuclear congression in wild-type and MPcfap53−/− embryos. (E) Graph shows quantification of observed cytokinesis. Numbers of embryos analyzed are indicated in the bars. Embryos were obtained from at least three independent pairs. An unpaired Student's t-test was used to test for significance. Scale bars: 20 μm. Schematics of embryonic development are adapted, with permission, from Kimmel et al. (1995). |
|
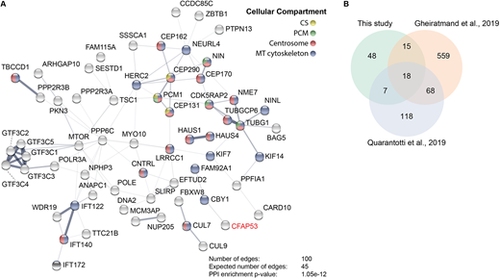
Cfap53 interacts with centrosomal, centriolar satellite and microtubule-associated proteins. (A) Network analysis using String-DB (Szklarczyk et al., 2015) from proteins identified by MS analysis after streptavidin-based purification using CFAP53 as bait. Only high-confidence hits from the MS dataset were used, which were obtained via stringent filtering steps on raw data (see Materials and Methods). Proteins shown are those with a significantly higher number of edges (representing protein-protein associations) than that expected stochastically. The thickness of the lines between the nodes does not represent strength or specificity of a given interaction, but represents the approximate confidence on a scale from zero to one of a found association being true, given the available evidence. Node colors represent cellular compartment of the indicated protein. (B) Venn diagram showing number of proteins identified in this study by bioGFP-Cfap53 pulldown with MS analysis overlapping with the centriolar satellite proteins that were identified by Gheiratmand et al. (2019) and Quarantotti et al. (2019). |
|
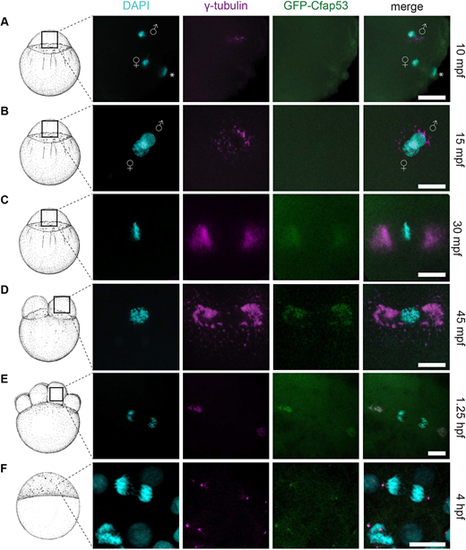
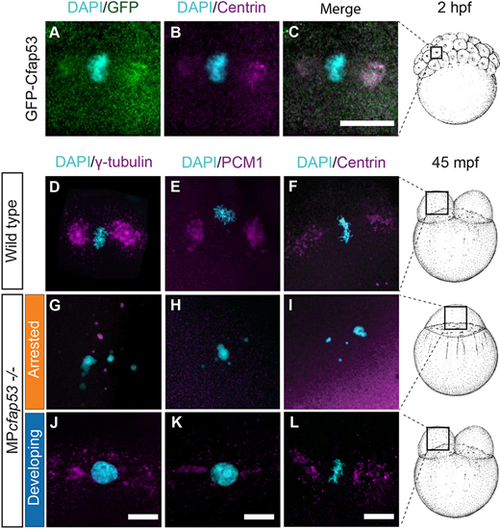
GFP-Cfap53 localization during cleavage stages. (A-F) Maximal projections of confocal stacks from Tg(ubi:GFP-cfap53) embryos immunolabelled for GFP, γ-tubulin and DAPI from 30 mpf until 4 hpf. (A) At 10 mpf, male and female pronuclei and the female polar body are visible. Some γ-tubulin foci around the male pronucleus can been observed, while GFP-Cfap53 has a diffuse localization (A, n=7). (B) At 15 mpf, both pronuclei are almost fused and some γ-tubulin can be observed around the nuclei, while GFP-Cfap53 localization is still diffuse (B, n=7). (C) At 30 mpf, chromosomes have aligned along the metaphase plate and strong γ-tubulin staining can be observed on either side of it. At this moment, GFP-Cfap53 also starts to localize to both sides of the metaphase plate, where it colocalizes with γ-tubulin (C, n=12). (D) At 45 mpf, the first cell division has completed and one of the two nuclei is shown. Both GFP-Cfap53 and γ-tubulin are visible in broad domains on both sides of the nucleus during prophase (D, n=8). (E) Single cell of an eight-cell stage blastula with segregating chromosomes during telophase of mitosis. GFP-Cfap53 and γ-tubulin colocalize in a more condensed region at both sites of the division plane (E, n=7). (F) Blastula cell in telophase during mitosis at the sphere stage (4 hpf). GFP-Cfap53 and γ-tubulin colocalize to a confined region on both sides of the division plane (F, n=1). Asterisks indicate polar bodies. Gender symbols indicate male or female pro-nucleus. Scale bars: 20 μm. Schematics of embryonic development are adapted, with permission, from Kimmel et al. (1995). |
|
Cfap53 is important for the localization of centrosomal components and centriolar satellites at the MTOC. (A-C, n=9) Maximal projections of confocal stacks from fixed Tg(ubi:GFP-cfap53) transgenic embryos immunolabeled for centrin at 2 hpf shows strong colocalization of GFP-Cfap53 with centrin during metaphase. (D-L) Maximal projections of confocal stacks from fixed wild-type or MPcfap53−/− embryos at 45 mpf immunolabeled for centrosomal components. γ-tubulin (D; n=8; G, n=15; J, n=3), PCM1 (E, n=7; H, n=15; K, n=3) and centrin (F, n=5; I, n=8; L, n=14) in wild-type embryos (D-F), MPcfap53−/− non-developing embryos (G-I) or MPcfap53−/− developing embryos (J-L) at 45 mpf fixed. Scale bars: 20 μm. Schematics of embryonic development are adapted, with permission, from Kimmel et al. (1995). |
|
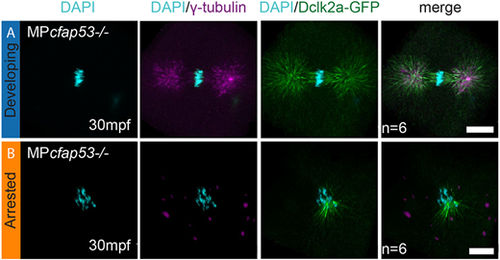
Cfap53 facilitates assembly of the zygotic spindle. (A,B) Maximal projections of confocal stacks on Tg(XlEef1a1:dclk2a-GFP)/MPcfap53−/− embryos at 30 mpf and immunolabeled for GFP, γ-tubulin and DAPI. While six embryos formed centrosomes and a normal mitotic spindle during the first zygotic cell division (A, n=6), six embryos failed to assemble the centrosomes and the mitotic spindle (B, n=6). Instead, microtubule originating from the chromosomes is observed in these embryos. Scale bars: 20 μm. |
|
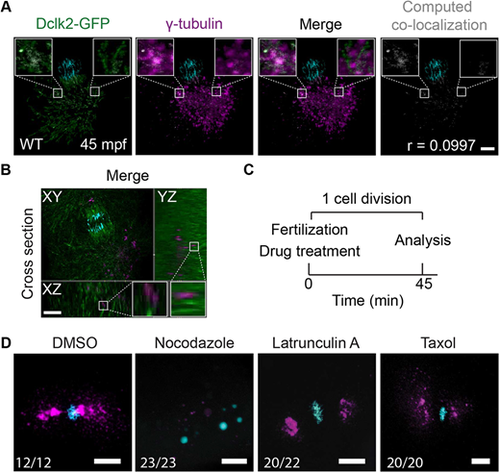
Microtubule-dependent γ-tubulin localization. (A,B) Maximal projections of confocal stacks from a Tg(Xla.Eef1a1:Dclk2a-GFP) embryo immunolabeled for DAPI (in cyan), Dclk2a-GFP (in green) and γ-tubulin (in magenta) at 45 mpf fixed during telophase. Colocalization of γ-tubulin and Dclk2a-GFP is shown in white in the merge. Computation of colocalization gives an average Pearson's coefficient of 0.1, indicating that there is very little colocalization of γ-tubulin and Dclk2a-GFP (A, n=5). Scale bar: 100 μm. (B) Optical cross-section through 45 mpf embryo immunostained for γ-tubulin and Dclk2a-GFP at the region of one of the centrosomes, which shows that γ-tubulin is localized directly adjacent to the microtubule bundles (B, n=5). Scale bar: 100 μm. White squares outline the areas shown in more detail. (C) Schematic overview of the drug treatment experiment (C), for which the results are shown in D. (D) Maximal projections of confocal stacks of embryos at 45 mpf immunostained with γ-tubulin (in magenta) and DAPI (in cyan) fixed during telophase. Nocodazole treatment (n=23) prevents γ-tubulin accumulation at the MTOC. Lantrunculin A (n=20) and taxol (n=20) treatments have no effect on γ-tubulin localization to the MTOC. Scale bars: 20 μm. |