- Title
-
A Subset of Oligodendrocyte Lineage Cells Interact With the Developing Dorsal Root Entry Zone During Its Genesis
- Authors
- Green, L.A., Gallant, R.M., Brandt, J.P., Nichols, E.L., Smith, C.J.
- Source
- Full text @ Front. Cell. Neurosci.
|
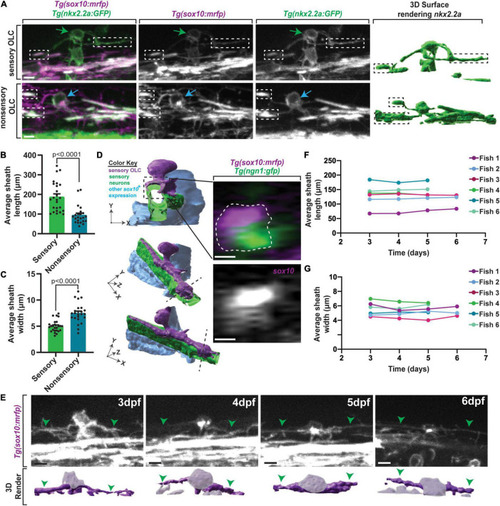
A distinct population of OPCs associates with sensory nerves. |
|
Sensory OLs maintain a distinct sheath profile. |
|
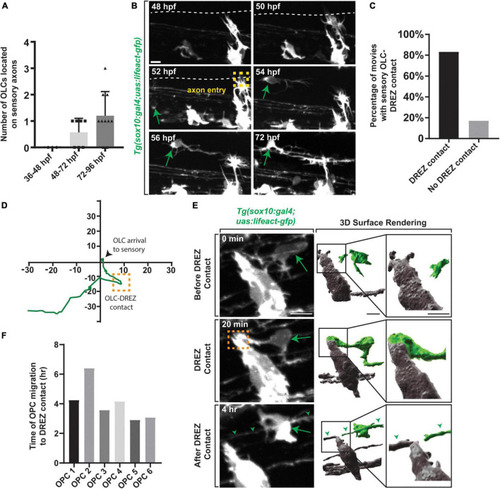
Oligodendrocyte progenitor cells contact the DREZ immediately following axon entry. |
|
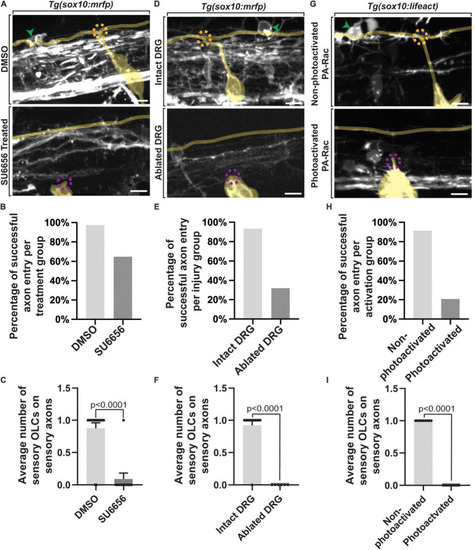
Failed axon entry does not result in sensory-related OLCs. |
|
Early axon entry promotes early OPC migration to sensory nerves. |
|
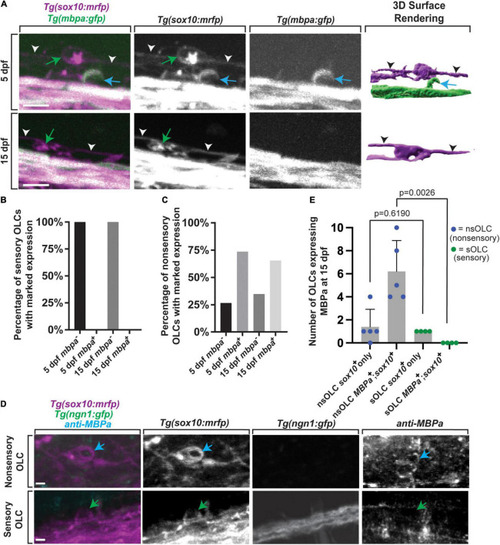
Sensory-related OLCs do not express typical OLC |
|
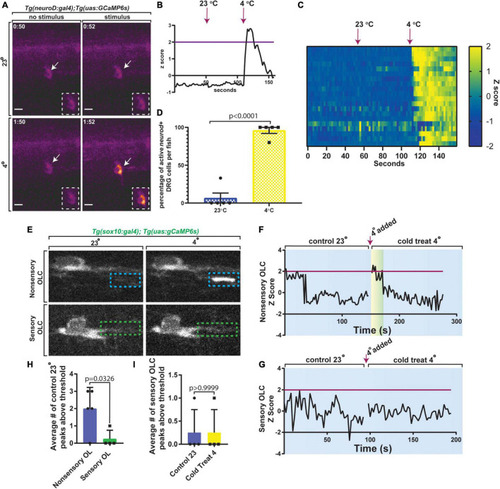
Sensory-related OLCs display distinct Ca2+ transients compared to non-sensory-related OLCs. |
|
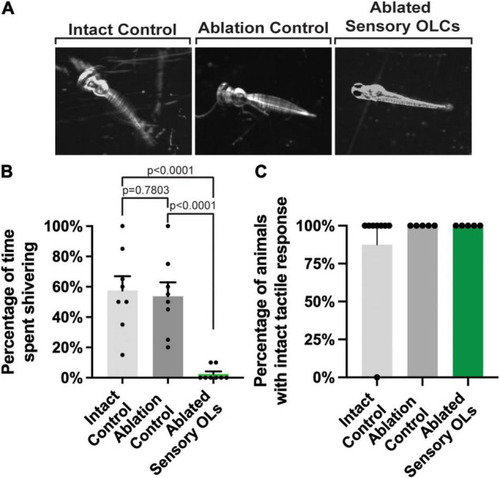
Sensory-related OLC ablation disrupts sensory behavior. |