- Title
-
Maternal Factors and Nodal Autoregulation Orchestrate Nodal Gene Expression for Embryonic Mesendoderm Induction in the Zebrafish
- Authors
- Xing, C., Shen, W., Gong, B., Li, Y., Yan, L., Meng, A.
- Source
- Full text @ Front Cell Dev Biol
|
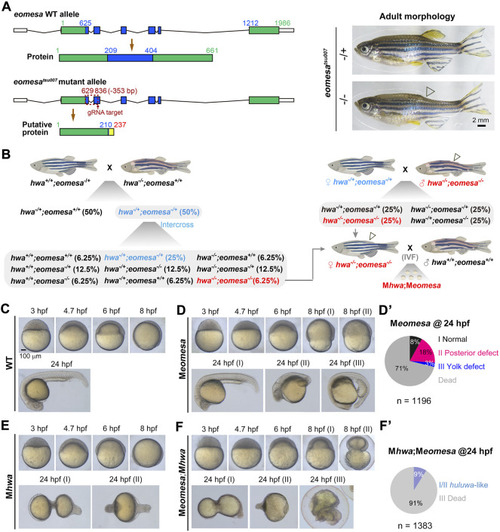
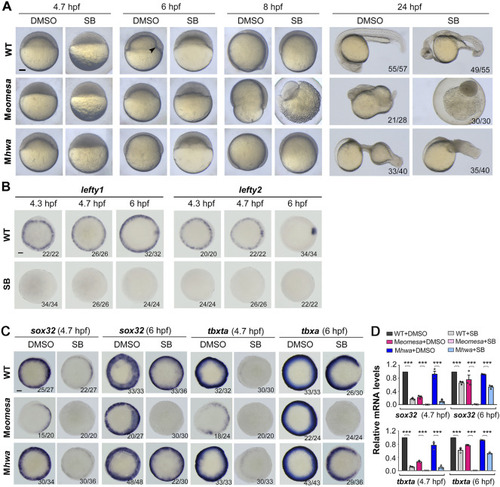
Phenotypes of different mutants at various stages. |
|
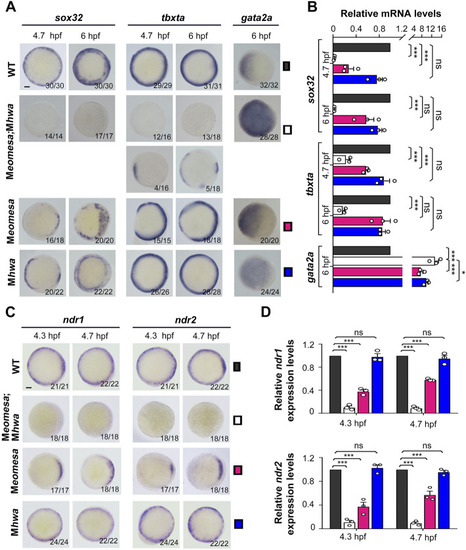
Expression patterns of mesendodermal markers and |
|
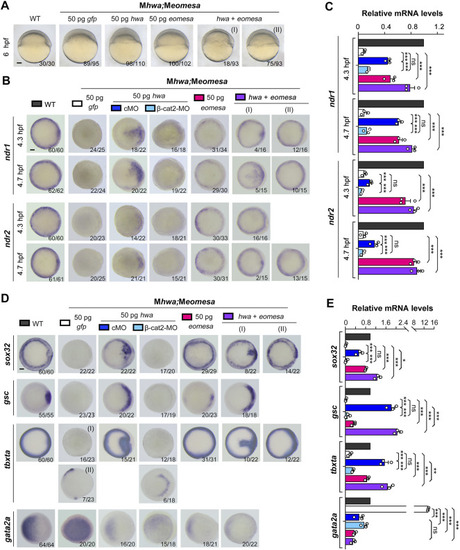
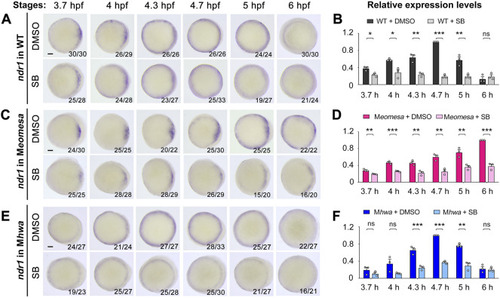
Induction of |
|
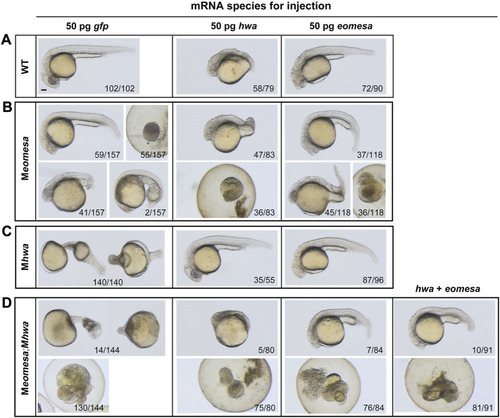
Overexpression effect of |
|
Responses of M |
|
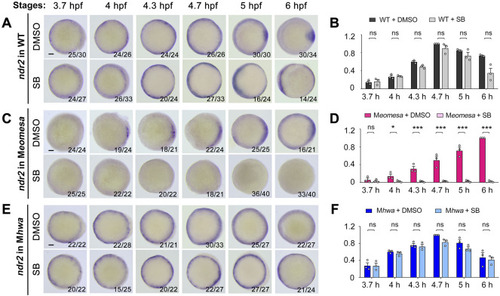
Effect of Nodal signaling inhibition on |
|
Effect of Nodal signaling inhibition on |
|
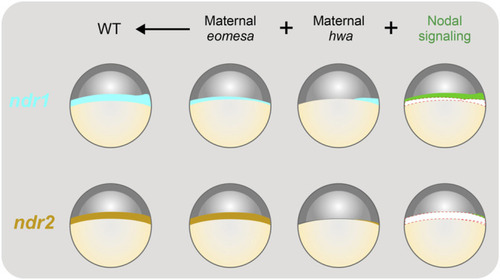
Illustration of contributions of maternal |