- Title
-
Vascular Injury in the Zebrafish Tail Modulates Blood Flow and Peak Wall Shear Stress to Restore Embryonic Circular Network
- Authors
- Baek, K.I., Chang, S.S., Chang, C.C., Roustaei, M., Ding, Y., Wang, Y., Chen, J., O'Donnell, R., Chen, H., Ashby, J.W., Xu, X., Mack, J.J., Cavallero, S., Roper, M., Hsiai, T.K.
- Source
- Full text @ Front Cardiovasc Med
|
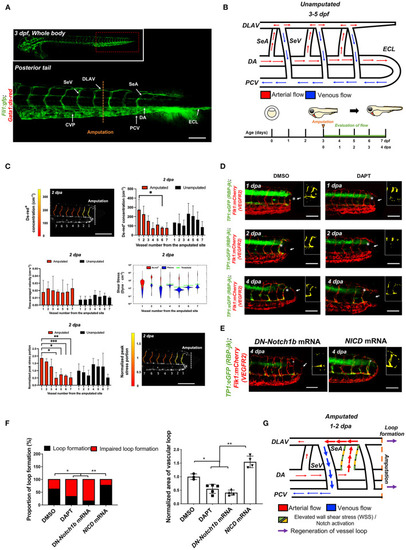
Tail amputation increases peak wall shear stress (WSS) in the segmental artery (SeA) closest to the amputation site to promote Notch-mediated dorsal longitudinal anastomotic vessel-posterior cardinal vein (DLAV-PCV) loop formation. |
|
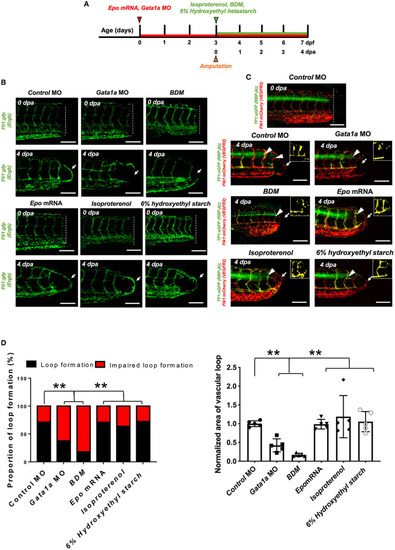
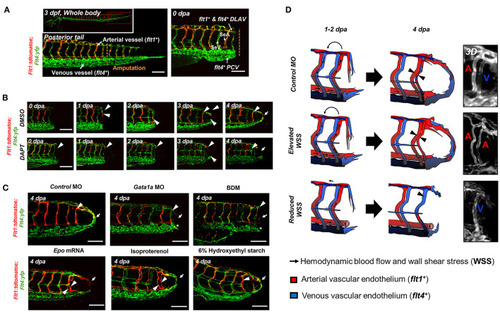
Changes in WSS modulate DLAV-PCV loop formation in a Notch-dependent manner. |
|
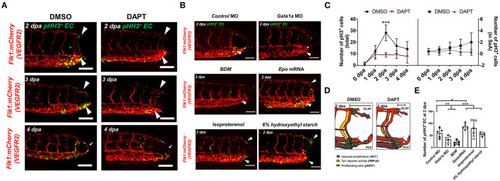
Figure 3. Amputation-mediated Notch signaling regulates EC proliferation in the DLAV and CVP during vascular loop formation. (A) DAPT treatment to inhibit Notch activity significantly reduced the number of EC proliferation (pHH3+EC, green) compared to DMSO-treated controls at 2 dpa (white arrowheads). Scale bar: 20 μm. (B) Gata1a MO injection or BDM treatment reduced, whereas epo mRNA and isoproterenol treatment increased pHH3+ EC at 2 dpa. (*p< 0.05, **p< 0.005, ***p< 0.0005 vs. control MO, n = 5 for each group). Scale bar: 20 μm. (C) Total numbers of endothelial pHH3+ EC in posterior tail segment and the caudal SeA were assessed to quantify Notch-dependent proliferation. 2 days of DAPT treatment resulted ~48% reduction in total number of pHH3+ EC, but not in the caudal SeA (**p< 0.005 vs. DMSO, n = 3 for DMSO, n = 5 per other groups). (D) Schematic representation of Notch-mediated pHH3+ EC during vascular loop formation. (E) Total pHH3+ EC in posterior tail were assessed to quantify WSS-dependent EC proliferation. Gata1a MO injection or BDM treatment reduced ~42 and ~65%, whereas epo mRNA and isoproterenol treatment increased pHH3+ EC by ~52 and ~39% respectively at 2 dpa. (*p< 0.05, **p< 0.005, ***p< 0.0005 vs. control MO, n = 5 for each group). Scale bar: 20 μm. |
|
WSS promotes Notch-mediated arterial network. |
|
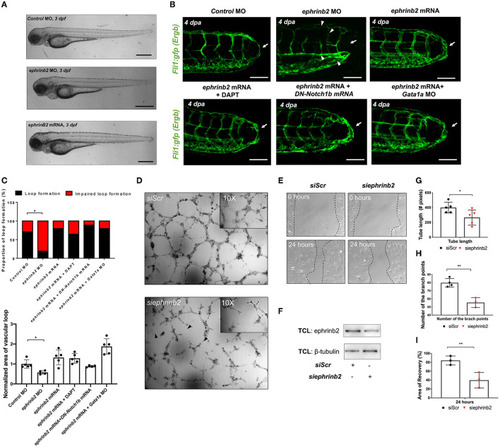
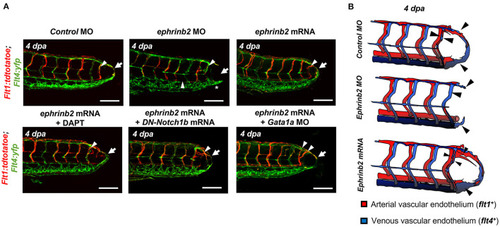
Ephrinb2 regulates vascular loop formation. |
|
WSS-responsive Notch-ephrinb2 pathway regulates arterial network formation. |
|
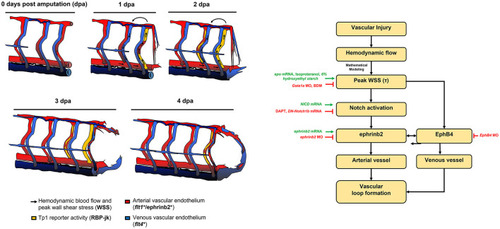
Schematic overviews of the proposed mechanisms underlying WSS-activated regrowth of the vascular loop. |