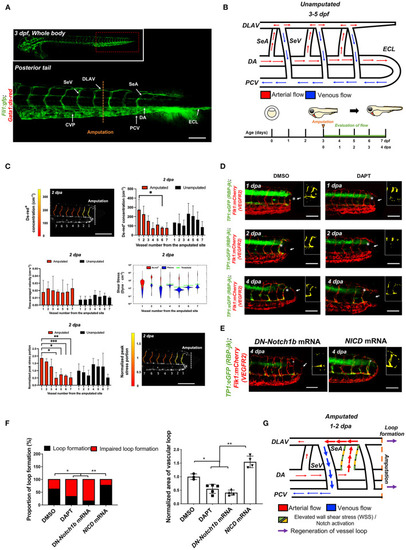
Tail amputation increases peak wall shear stress (WSS) in the segmental artery (SeA) closest to the amputation site to promote Notch-mediated dorsal longitudinal anastomotic vessel-posterior cardinal vein (DLAV-PCV) loop formation. (A) Anatomy of tail vasculature in Tg(fli1:gfp; gata1:ds-red) embryos; Scale bar: 100 μm. (B) Wiring diagram illustrate embryonic circulation in zebrafish tail. In the caudal vascular network, dorsal aorta (DA) forms an embryonic circulatory loop (ECL) with PCV (white arrow), and venous flow in the DLAV and venous segmental vessel (SeV) drain into PCV (white arrowheads). Experimental design: At 3 days post fertilization (dpf), embryos were randomly chosen for tail amputation (~100 μm of posterior tail segment). Hemodynamic WSS was evaluated for 4 consecutive days. (C) At 2 dpa, ds-red+ concentration (cm−1, number of ds-red+ per unit length of SeA) in the amputated site increased significantly compared to unamputated embryos (Mean ± standard deviation across the vessels in 3 unamputated embryos, averaged over 3.6 mins). Time-averaged velocity (cm·s−1) remained unchanged. The distribution of WSS exerted by ds-red+ (red) is shifted upward compared to that exerted by plasma (blue), giving intermittent rises of WSS as each ds-red+ passes. Although this separation is present in every SeA, the portion of ECs (red area) experiencing WSS from ds-red+ is higher in the proximal SeAs than in the distal SeAs, leading to a higher peak stress portion. The areas scale with but are not equal to the portion of endothelial cells (ECs) that experience stress from ds-red+ and plasma. Green line shows the 0.975 percentile of WSS from plasma, which is used as an activation threshold. For the calculation of WSS, see Methods. The increase in viscosity resulted WSS to exceed activation threshold in the site of amputation. The threshold is set to 0.975 percentile of plasma shear stress across all intersegmental vessels, and ranges from 1.1 to 5.4 dyne·cm−2. (D) In the transgenic Tg(tp1:gfp; flk1: mcherry) line, vehicle Dimethyl sulfoxide (DMSO)-treated zebrafish showed prominent endothelial tp1 activity in the proximal SeA (*asterisk, overlapped yellow) and formed a new vascular loop between the DLAV and PCV at 4 dpa (white arrow). Pharmacological γ-secretase inhibitor (DAPT) treatment (100 μM) attenuated endothelial tp1 activity in the amputated site and neighboring SeA and impaired regeneration of the loop by 4 dpa (white arrow). Scale bar: 20 μm. (E) Transient suppression of Notch activity via DN-Notch1b mRNA, but not Notch Intracellular Cytoplasmic Domain (NICD) mRNA, impaired vascular loop formation. Scale bar: 20 μm. (F) Quantification of the proportion of embryos exhibiting loop formation and normalized area of vascular loop (*p< 0.05 vs. DMSO, **p< 0.005 vs. NICD mRNA, n = 17 for DMSO, n = 20 for DAPT, DN-Notch1b and NICD mRNAs to assess proportion of loop formation, n = 3 for DMSO, n = 5 for DAPT, DN-Notch1b and NICD mRNAs to assess area of vascular loop). (G) Wiring diagrams illustrate amputation-mediated changes in blood flow and hemodynamic WSS. SeA, Arterial segmental vessel; SeV, Venous segmental vessel; DLAV, Dorsal longitudinal anastomotic vessel; PCV, Posterior cardinal vein; DA, Dorsal aorta; CVP, Caudal vein capillary plexus; ECL, Embryonic circulatory loop. ***p < 0.0005.
|

