- Title
-
Reactivation of the Neurogenic Niche in the Adult Zebrafish Statoacoustic Ganglion Following a Mechanical Lesion
- Authors
- Schwarzer, S., Rekhade, D.R., Machate, A., Spieß, S., Geffarth, M., Ezhkova, D., Hans, S.
- Source
- Full text @ Front Cell Dev Biol
|
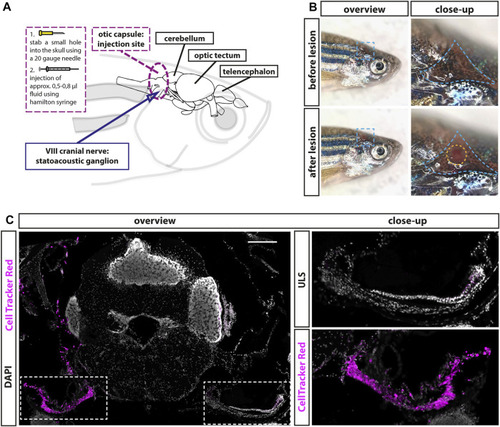
Establishment of a lesion paradigm of the adult zebrafish statoacoustic ganglion (SAG). |
|
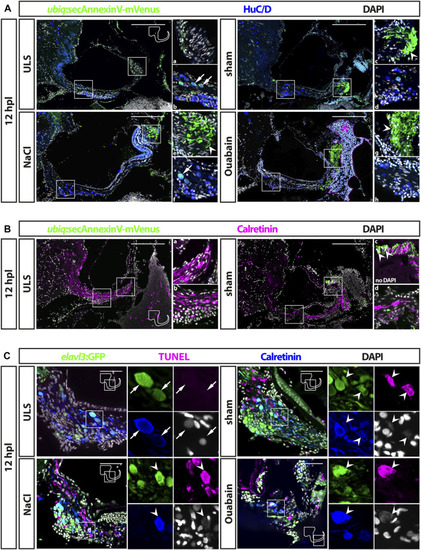
Cell death in the adult zebrafish SAG upon lesion. |
|
Accumulation of leukocytes in the adult zebrafish SAG upon lesion. Antibody staining against the pan-leukocyte marker L-plastin and GFP in the SAG of transgenic |
|
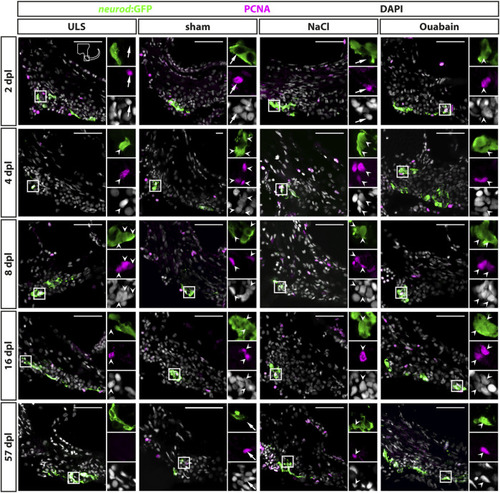
Reactive proliferation of |
|
Quantification of reactive proliferation of |
|
Generation of new sensory neurons from the |
|
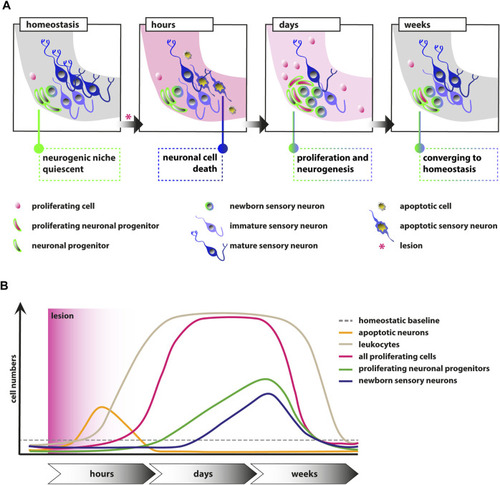
FIGURE 7. Schematic illustrations of the events in the adult zebrafish SAG upon lesion. (A) Scheme depicting the cellular events within the neurogenic niche. During homeostasis, the neurogenic niche of the adult zebrafish SAG is quiescent in regard to proliferation and shows only very rare neurogenesis. Upon lesion, mature sensory neurons as well as other marker-negative cells undergo apoptosis within several hours. Within days, reactive proliferation of marker-negative and neuronal progenitor cells is observed accompanied by reactive neurogenesis from the neuronal progenitor pool. Reactive proliferation and reactive neurogenesis continue over the next weeks until previous homeostatic levels are reached. (B) Scheme of the temporal order of events. During homeostasis, only basal levels of proliferating marker-negative cells, proliferating neuronal progenitors, and leukocytes are present in the neurogenic niche of the SAG. In addition, only basal levels of newborn neurons derived from the neuronal progenitors and no apoptotic cells are found. Upon lesion, a strong increase in the number of apoptotic cells and, in particular, apoptotic sensory neurons is observed, which is also resolved rapidly. Also within hours, a massive accumulation of leukocytes occurs, which persists for several weeks. Within days, the number of proliferating cells increases significantly within the neurogenic niche of the SAG and remains high for a couple of weeks until it returns to lower levels. Following the general gain in proliferation, the number of proliferating neuronal progenitors is also significantly increased for approximately 2–3 weeks until it returns to baseline levels. Onset of proliferation of neuronal progenitors is accompanied by increased production of newborn neurons, which remains high for approximately 2–3 weeks until it returns to homeostatic levels. |