- Title
-
Svep1 stabilizes developmental vascular anastomosis in reduced flow conditions
- Authors
- Coxam, B., Collins, R.T., Hußmann, M., Huisman, Y., Meier, K., Jung, S., Bartels-Klein, E., Szymborska, A., Finotto, L., Helker, C.S.M., Stainier, D.Y.R., Schulte-Merker, S., Gerhardt, H.
- Source
- Full text @ Development
|
|
|
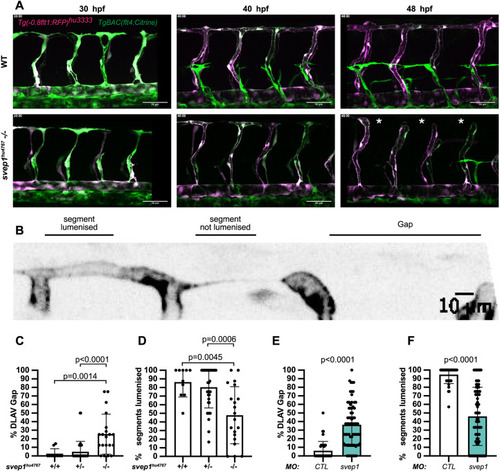
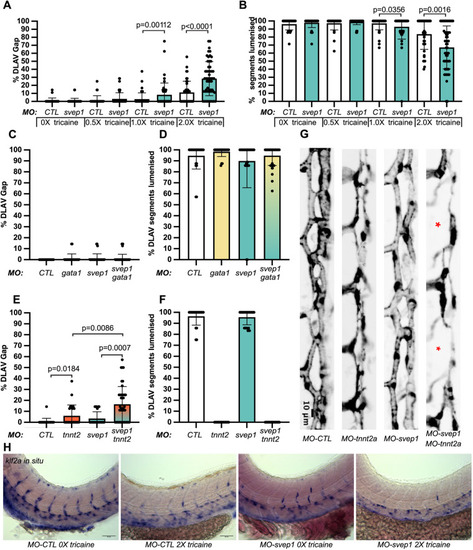
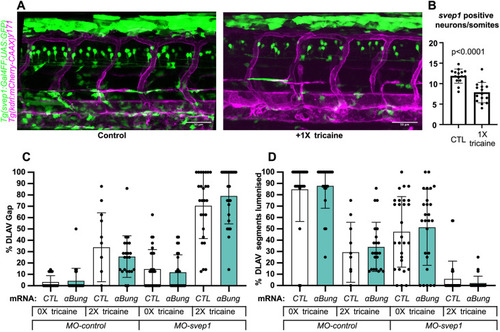
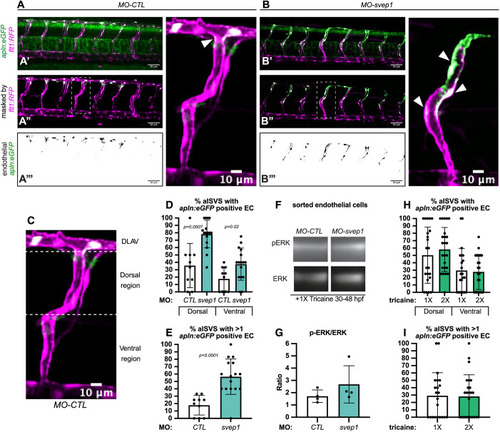
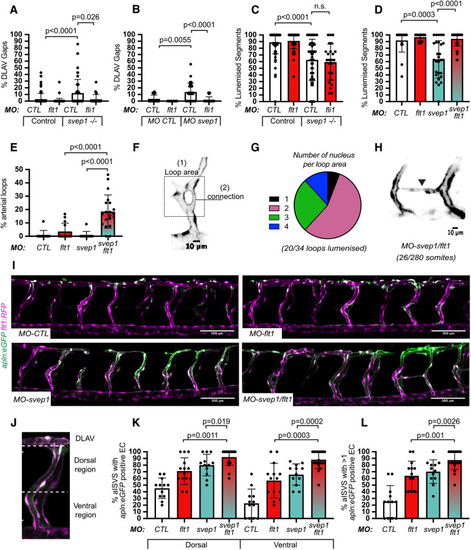
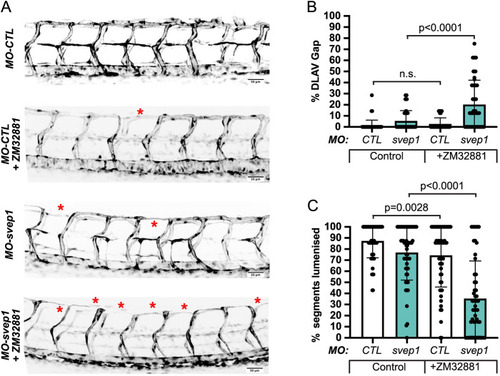
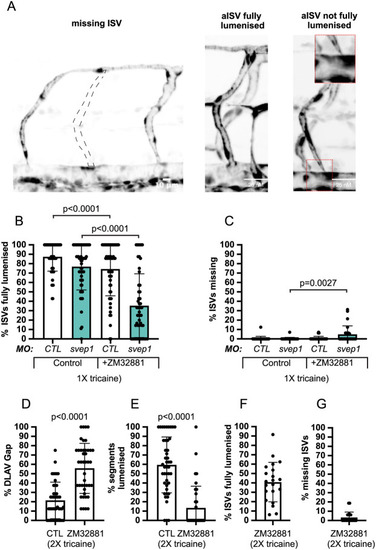
svep1 loss-of-function sensitises angiogenic remodelling to reduced blood flow. (A) Bilateral quantifications of the percentage of gaps in the DLAV at 48 hpf in embryos injected with MO-CTL (5 ng) and MO-svep1 (5 ng) and treated with 0× (n=14 MO-CTL, n=20 MO-svep1), 0.5× (n=16 MO-CTL, n=24 MO-svep1), 1× (n=22 MO-CTL, n=27 MO-svep1) or 2× (n=21 MO-CTL, n=27 MO-svep1) from 30 to 48 hpf (N=3). (B) Bilateral quantifications of the percentage of lumenised segments in the DLAV at 48 hpf in embryos injected with MO-CTL (5 ng) and MO-svep1 (5 ng) and treated with 0× (n=14 MO-CTL, n=20 MO-svep1), 0.5× (n=16 MO-CTL, n=24 MO-svep1), 1× (n=22 MO-CTL, n=27 MO-svep1) or 2× (n=21 MO-CTL, n=27 MO-svep1) from 30 to 48 hpf (N=3). (C) Bilateral quantifications of the percentage of gaps in the DLAV at 48 hpf in embryos injected with MO-CTL (5 ng) (n=13), MO-gata1 (8 ng) (n=12), MO-svep1 (5 ng) (n=16) and MO-gata1 (8 ng)/MO-svep1 (5 ng) (n=25) (N=3). (D) Bilateral quantifications of the percentage of lumenised segments in the DLAV at 48 hpf in embryos injected with MO-CTL (5 ng) (n=13), MO-gata1 (8 ng) (n=12), MO-svep1 (5 ng) (n=16) and MO-gata1 (8 ng)/MO-svep1 (5 ng) (n=25) (N=3). (E) Bilateral quantifications of the percentage of gaps in the DLAV at 48 hpf in embryos injected with MO-CTL (5 ng) (n=11), MO-tnnt2a (4 ng) (n=12), MO-svep1 (5 ng) (n=12) and MO-tnnt2a (4 ng)/MO-svep1 (5 ng) (n=21) (N=3). (F) Bilateral quantifications of the percentage of lumenised segments in the DLAV at 48 hpf in embryos injected with MO-CTL (5 ng) (n=11), MO-tnnt2a (8 ng) (n=12), MO-svep1 (5 ng) (n=12) and MO-tnnt2a (4 ng)/MO-svep1 (5 ng) (n=21) (N=3). (G) Maximum intensity projection of dorsal view of the DLAV from representative embryos injected with MO-CTL (5 ng) (n=11), MO-tnnt2a (8 ng) (n=12), MO-svep1 (5 ng) (n=12) and MO-tnnt2a (4 ng)/MO-svep1 (5 ng) (n=21) embryos at 48 hpf. Red asterisks indicate gaps. (H) Bright-field images of klf2a in situ hybridisation of 48 hpf embryos injected with MO-CTL or MO-svep1 and treated with 0× or 2× tricaine from 30 to 48 hpf. Data are mean±s.d. Mann–Whitney test. Scale bars: 10 μm (G); 50 μm (H). |
|
|
|
|
|
|
|
|
|
|