- Title
-
Zebrafish Model-Based Assessment of Indoxyl Sulfate-Induced Oxidative Stress and Its Impact on Renal and Cardiac Development
- Authors
- Tang, P.W., Wu, P.H., Lin, Y.T., Chiu, C.H., Cheng, T.L., Guan, W.H., Lin, H.Y., Lee, K.T., Chen, Y.H., Chiu, C.C., Liu, W.
- Source
- Full text @ Antioxidants (Basel)
|
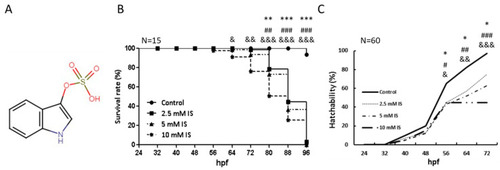
Toxicity profile of IS as indicated by the survival and hatchability of zebrafish embryos (A) Structure of IS. (B) Time-course plot of embryo survival in the control group vs. the IS group at 96 h. (C) Hatchability of zebrafish after IS treatment. hpf: hours postfertilization. (B,C) were statistically analyzed using Fisher’s exact test. * p < 0.05, ** p < 0.001 and *** p < 0.0001, the 2.5 mM IS group compared with the control group. # p < 0.05, ## p < 0.001 and ### p < 0.0001, the 5 mM IS group compared with the control group. & p < 0.05, && p < 0.001 and &&& p < 0.0001, the 10 mM IS group compared with the control group. N indicates the embryo number for each group. |
|
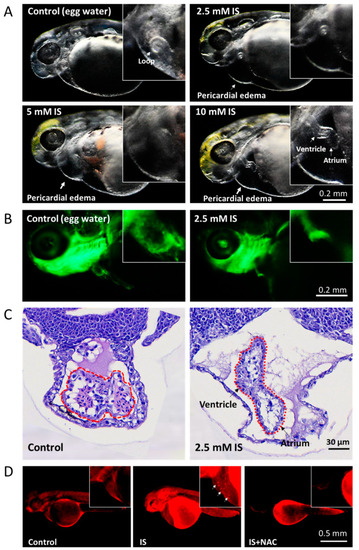
Assessment of the effect of IS on cardiac development in zebrafish embryos. (A) Morphological changes in the heart. The results showed that IS exposure causes cardiac dysmorphology and pericardial edema in zebrafish embryos. Furthermore, the atria and ventricles of the heart were elongated and separated. Morphology was assessed visually using a stereomicroscope (Leica MZ10 F). (B) Tg(fli1:GFP) zebrafish embryos were treated with 2.5 mM IS. (C) HE staining. Zebrafish embryos treated with 2.5 mM IS were fixed in 10% formalin for one day, immersed in 1% agarose, paraffin-embedded and stained with HE. The stained tissues were observed under a microscope. The dotted lines indicate the developing heart. Compared to the control condition, IS exposure caused elongation of the heart and distal separation of the ventricle and atrium. (D) Representative results for ROS production in pericardial tissues using DHE staining. The white arrows indicate the signals where ROS accumulated in the pericardium. Control embryos were soaked in egg water (60 μg/mL sea salt) without IS treatment. |
|
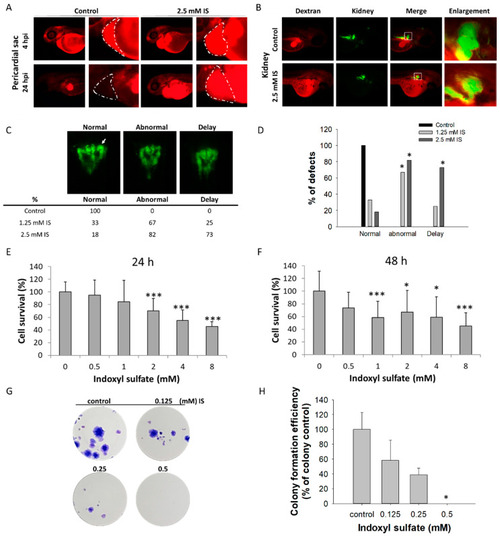
Impact of IS on renal development. The results of the zebrafish assay are shown in (A–D); those obtained with the HEK293T cell line are shown in (E–H). After treatment with IS, dextran clearance was reduced, and tubular transport function was disrupted. IS- and vehicle-treated larval zebrafish were injected with 10 kDa rhodamine-labeled dextran at 48 hpf, and the fluorescence of each embryo was measured after 4 and 24 h, respectively. (A) Visual comparison of rhodamine-labeled dextran clearance in two individual fish after IS treatment. The impact of IS exposure on renal clearance of dextran was assessed to determine renal function. (B) Morphological changes in renal development. ■ Red fluorescence indicates rhodamine-labeled dextran; ■ green fluorescence indicates kidney tissue. (C) Results of renal defect assessment. The white arrows indicate perinephric tubules. (D) Quantitative results of (C) Fisher’s exact test. An MTS-based assay was used to assess the viability of human HEK293T cells treated with IS for (E) 24 h and (F) 48 h. The results of the colony formation assay are shown in (G) HEK293T cells were treated with the indicated concentrations of IS (from 0.125 to 0.5 μM) for 14 days. Afterward, the cells were fixed in 4% paraformaldehyde and stained with Giemsa. (H) Quantitative analysis of (G) the colony formation assay. * p < 0.05, *** p < 0.005. |
|
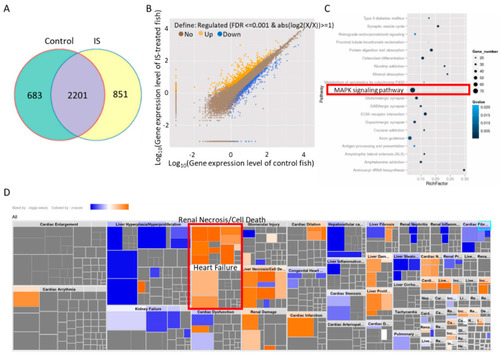
KEGG enrichment and IPA of differentially expressed genes in zebrafish. (A) The Venn diagram shows the coexpressed genes between IS-treated and control embryos. (B) Scatter plots of all expressed genes between IS-treated and control zebrafish embryos plotted as log10 (gene expression level with FDR ≤ 0.001 & abs(log2(X/X)) ≥ 1). (C) Scatter plot of KEGG enrichment analysis of the differentially expressed genes between IS-treated and control fish embryos. The red border indicates the candidate MAPK pathway. (D) Toxicological functions of the differentially expressed genes related to IS exposure. |
|
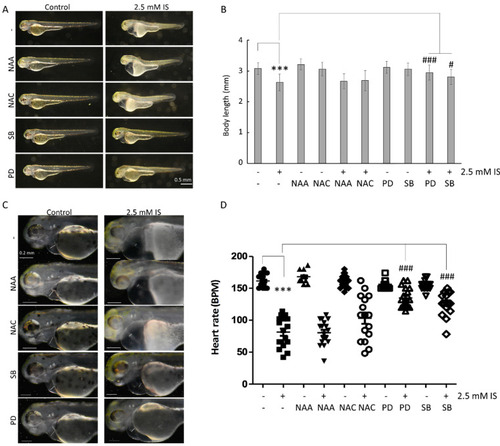
Inhibition of MAPK pathways attenuates the impact of IS on the embryonic development of zebrafish. (A) Changes in embryo body length. (B) Quantitative results for (A). (C) Survival analysis. (D) Analysis of heart rate. SB: SB203580, a MAPK p38 inhibitor; PD: PD98059, a MAPK ERK inhibitor. NAA was used as a negative control for ROS scavenging. *** p < 0.001 compared with the control group. # p < 0.05 and ### p < 0.001 compared with the group with IS exposure. |
|
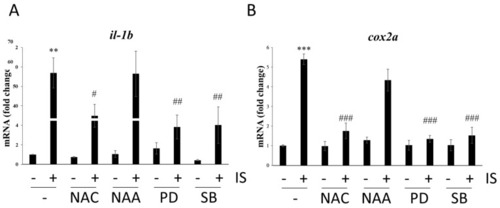
Effects of ROS scavengers and MAPK inhibitors on the mRNA expression of proinflammatory genes. qPCR-based assessment of the mRNA levels of (A) il-1b and (B) cox2a. ** p < 0.01 and *** p < 0.001 compared with the control group. # p < 0.05, ## p < 0.01 and ### p < 0.001 compared with the IS-treated group. |
|
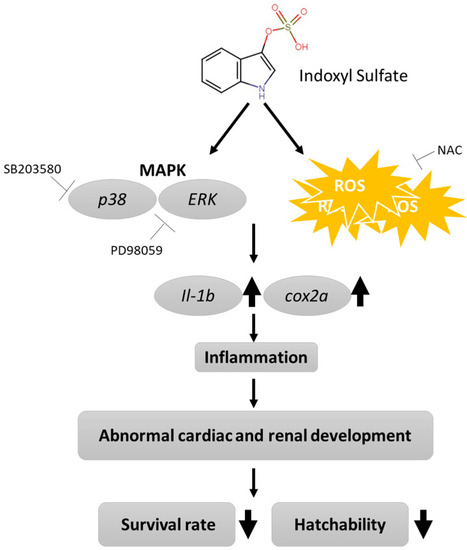
Proposed mechanism of IS-induced morphogenesis and physiological abnormalities during development in zebrafish embryos. |