- Title
-
Ca2+-imaging and photo-manipulation of the simple gut of zebrafish larvae in vivo
- Authors
- Okamoto, S.I., Hatta, K.
- Source
- Full text @ Sci. Rep.
|
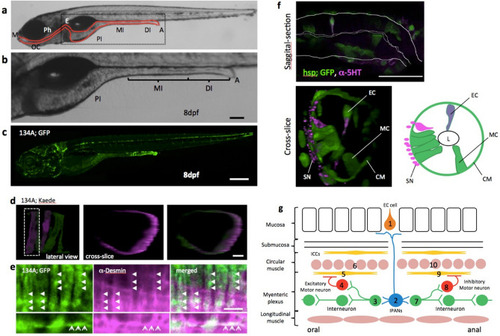
Simple structure of the zebrafish gut. (a) Lateral view of zebrafish larva at 8 dpf, indicating the principal digestive tract. The intestine is divided into three parts: proximal intestine (PI), middle intestine (MI), and the distal part of the intestine (DI)10. The PI is also referred to as the intestinal bulb and is thought to have similar functions as the stomach, which zebrafish do not have. The MI is thought to absorb nutrients and plays a role in mucosal immunity. The DI is analogous to the colon. M mouth, OC oral cavity, Ph pharynx, E esophagus, PI proximal intestine, MI middle intestine, DI distal intestine, A anus. (b) A higher-magnification image of the area indicated by the square in (a). Scale bar, 50 μm. (c) Lateral view of SAGFF(LF)134A; Tg(UAS: GFP) at 8 dpf. Scale bar, 200 μm. (d) Live confocal image of individual circular smooth muscles visualized by green to red photoconversion of Kaede fluorescence in SAGFF(LF)134A; Tg(UAS: Kaede) at 5 dpf. Lateral projection view shows two muscles photoconverted. Left side view. Cross-slice view of the dotted area shows a single red muscle. Scale bar, 10 μm. (e) Saggital sections of a confocal image of SAGFF(LF)134A; Tg(UAS: GFP) at 8 dpf stained with anti-Desmin antibody. Upper panels demonstrate Desmin filaments (in magenta, shown by triangles) located inside of each circular smooth muscles expressing GFP. Lower panels show an example of occasional appearance of longitudinal muscle cell expressing GFP, containing Desmin filaments (arrows). Scale bar, 10 μm. (f) Saggital section and cross-slice of the gut, indicating a variety of cell types visualized at 5 dpf in Tg(hsp70: Gal4); Tg(UAS: GFP) by heat shock at 3 dpf. Double immunostaining for anti-GFP (green) and anti-5-HT (magenta). EC serotonergic endochromaffin cells, MC mucosal cells, L lumen, CM circular muscle, SN neurites of serotonergic neurons. Scale bar, 50 μm. (g) Schematic diagram showing the hypothetical circuitry for the peristaltic reflex in the zebrafish gut. 1, endochromaffin cell; 2, intrinsic primary afferent cell; 3, ascending interneuron; 4, excitatory motoneuron; 5, 9, interstitial cells of Cajal; 6, 10, circular smooth muscles; 7, descending interneuron; 8, inhibitory motoneuron. |
|
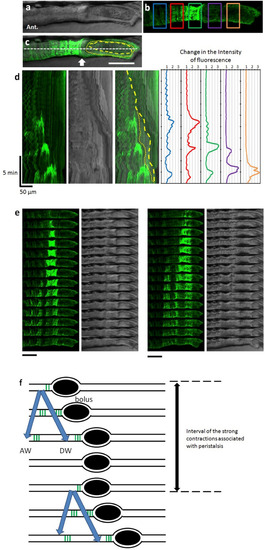
Peristaltic reflex visualized by Ca2+ imaging of circular smooth muscles. (a–c) Live confocal image of the lateral view of SAGFF(LF)134A; Tg(UAS: GCaMP3) at 8 dpf. Bright field image (a), GCaMP3 fluorescence (green) (b), and merged image (c). In (b), measurement locations are shown in rectangles. In (c), the yellow dashed line represents the outline of the bolus. The yellow dotted line represents the outline of the liquid surrounding the bolus. Scale bar, 50 μm. (d) Kymograph of GCaMP3 fluorescence, bright field image and merged image at the site noted by the white dashed line in (c), and time course of the fluorescence intensity at each site noted by the five squares in (b). (e) Time-lapse series of the second (left) and third (right) events in (d). Scale bars, 50 μm. Interval, 11 s. (f) Schematic diagrams showing Ca2+ events in the circular muscles at the peristaltic movement. Black ellipses denote bolus. Also see Supplementary video 1. |
|
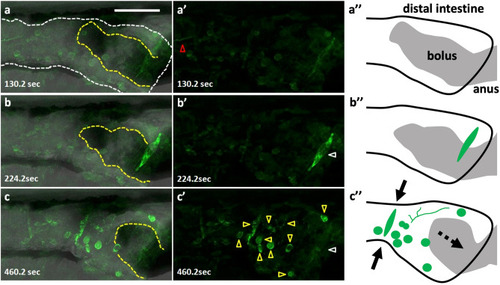
Peristaltic reflex visualized by Ca2+ imaging of a variety of cell types including putative enteric neurons and circular smooth muscles. Live images of putative enteric neurons and circular smooth muscles expressing GCaMP3 at 8 dpf in Tg(hsp70: Gal4); Tg(UAS: GCaMP3), heat-shocked at 6 dpf. The passage of the bolus was blocked at the anus by agarose gel, in an artificial condition of constipation. (a–a’’) the phase when most of the activity is arrested but an activated fiber with a distinct rhythm extends from the oral side (red triangle). (b–b’’) the phase when only the putative circular muscles at the anal side (white triangle) located on the bolus (yellow dashed lines) are activated. (c–c’’) the phase when the putative circular muscles located at the oral side of the bolus are activated together with most of the putative neurons and axons in the view (yellow triangles) (a–c), merged image; (a’–c’), fluorescence; (a’’–c’’), schematic drawings. Solid arrows indicate a contraction of the gut and a dashed arrow, the movement of the bolus. Lateral views. Anterior to the left. Scale bar, 50 μm. Also see Supplementary video 2. |
|
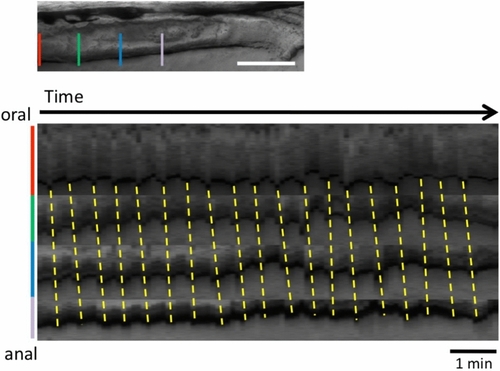
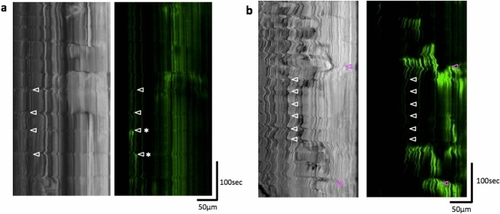
Posterior propagation of the regular movements, that is distinct from the peristaltic reflex, observed in the distal intestine of zebrafish larva at 8 dpf with a bolus. Kymographs of the bright field movie at four positions in the distal intestine (colored lines in the upper panel) along the anterior–posterior axis, from the data shown in Fig. 2 (20 min duration; Also see Supplementary video 1). Dotted lines demonstrate that the waves of periodic contractions propagate in the caudal direction. These regular movements may correspond to slow waves. See the text for the detail. Scale bar, 50 μm. |
|
Regular movements observed in the distal intestine at 4 and 7 dpf and peristaltic reflex-like activity observed at 7 dpf without feeding. Kymgraphs of the bright field and GCaMP3 fluorescence movies, at 4 dpf (a) and 7 dpf (b). Regular movements (white triangles) were not always associated with Ca2+ events in the circular smooth muscles, although weak Ca2+ events could be sometimes associated with the regular movements, as indicated by asterisks in (a). On the other hand, peristaltic reflex-like movement (magenta triangles) which was sometimes observed without feeding was associated with strong Ca2+ events in the circular smooth muscles. |
|
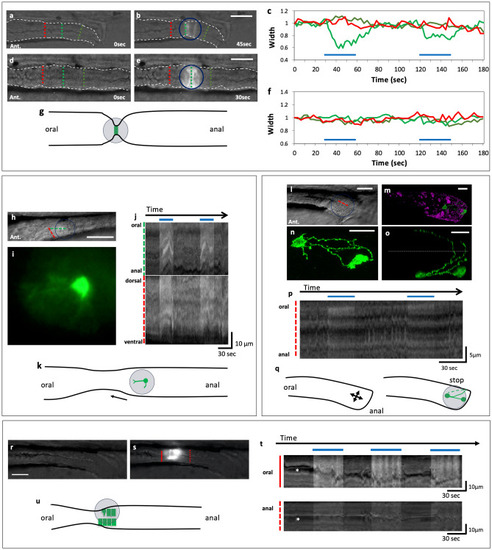
Optogenetic activation of a small number of smooth muscle cells, neurons or endodermal cells could induce or arrest gut movement. (a–g) Activation of circular smooth muscles caused local contraction of the gut. Blue-light irradiation (indicated by a circle) of circular muscles expressing ChR2-eYFP in SAGFF(LF)134A; Tg(UAS: ChR2-eYFP) (a,b) and the control expressing GFP in SAGFF(LF)134A; Tg(UAS: GFP) (d,e) in the posterior gut at 8 dpf. The changes in the width of the gut (c,f). Green lines indicate the position of the measurement in the spot of blue light; red lines at the oral side of the spot; yellowgreen lines at the anal side. Blue lines in (c,f) indicate the periods when blue light was applied. Schematic representation of the gut movement induced by the spot light (g). Lateral views. Ant., anterior. Scale bars, 50 μm. Also see Supplementary videos 3 and 4. (h–k) Activation of a single neuron could induce contraction at the oral side of the neuron. Live view of the midgut (h) and the neuron expressing ChR2-eYFP in a spot of the blue light, extending one of the neurites anteriorly (i) and two kymographs of (g) at the oral-to-anal (green) and the dorsal-to-ventral (red) dashed lines (j). Schematic representation of the gut movement induced by the spot light (k). Scale bars, 50 μm for h and 10 μm for i. Also see Supplementary video 5. (l–q) Activation of two neurons located near the anus could cause the arrest of the local gut movement at 8 dpf of Tg(hsp70: Gal4); Tg(UAS: ChR2-eYFP). Live image of the gut (l). Ant., anterior. Double immunostaining for anti-GFP indicating the cells expressing ChR2-eYFP (green) and anti-5-HT (magenta) (m). Enlarged views of these neurons at lateral view (n) and dorsal view (o) indicating that some axons are extending across the dorsal midline of the gut. Dotted line indicates the dorsal midline. Kymograph at the dashed red line in (l). Schematic representation of the arrest of the gut movement induced by the spot of blue light (q). Scale bars, 20 μm. Also see Supplementary video 6. (r–u) Activation of cells in the endodermal cell layer could induce a peristaltic reflex-like movement (a local constriction of the gut and active movement at the oral part) at 5 dpf of Tg(hsp70: Gal4); Tg(UAS: ChR2-eYFP). The same gut before (r) and during (s) the irradiation with the spot of blue light. Kymographs at the solid and dashed red lines in (s, t). Schematic representation of the gut movement induced with the light (u). Asterisks indicate the lumen of the gut. Arrows indicate the movement of the gut. Scale bars, 20 μm. Also see Supplementary video 7. |