- Title
-
Radiation dose enhancement using gold nanoparticles with a diamond linear accelerator target: a multiple cell type analysis
- Authors
- Piccolo, O., Lincoln, J.D., Melong, N., Orr, B.C., Fernandez, N.R., Borsavage, J., Berman, J.N., Robar, J., Ha, M.N.
- Source
- Full text @ Sci. Rep.
|
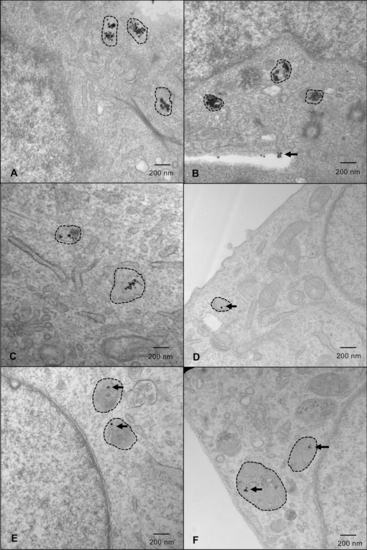
HNC cells demonstrate the greatest uptake of GNPs in lysosomes. Representative images of cells labelled with 25 µM GNPs. Lysosomes are indicated with hashed outlines. (a) FaDu; (b) HSC-3; (c) Detroit-562; (d) SK-N-AS; (e) Panc1; and (f) A673 cells. Cells were fixed approximately 36 h after GNP-labelling (approximate radiation timepoint) and images were captured by a transmission electron microscope. Imaging was done in triplicate—5 images were captured for each sample, with 3 samples/replicate, and 3 replicates in total. HNC head and neck cancer, GNPs gold nanoparticles. |
|
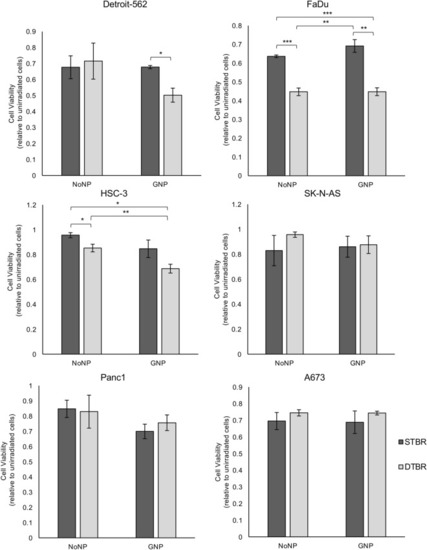
GNP-labeled HNCCs irradiated with the DTB show reduced viability 3 days post-irradiation. The alamarBlue viability assay was used to determine the levels of oxidative phosphorylation in cells irradiated with the STB or DTB and labelled with GNPs or NoNPs, 3 days after radiation. Detroit-562, FaDu, and HSC-3 HNCC lines demonstrated a significant reduction in cell viability following 8 Gy irradiation with the DTB in cells labelled with GNP compared to NoNP-labelled cells with STBR (p = 0.018, p = 0.004, p = 0.003, respectively). NoNP FaDu cells irradiated with the DTB demonstrated decreased viability compared to NoNP and GNP cells with STBR (p = 0.001 and p = 0.003, respectively). Similarly, NoNP HSC-3 cells irradiated with the DTB demonstrated a reduction in viability compared to NoNP cells with STBR (p = 0.049). With DTBR, GNP-labelled HSC-3 cells decreased compared to NoNP cells (p = 0.024). SK-N-AS cells, Panc1 cells, and A673 cells demonstrated no significant differences in viability. Cell viability values are made relative to control cells that were not irradiated and are presented as the fold change means ± standard error of the mean, p* < 0.05, p** < 0.01, p*** < 0.001 for significant decrease in cell viability. Significance between groups was tested using one-way analysis of variance (ANOVA) with a Tukey multiple comparisons test (n = 3; 10 wells per group/replicate). GNP gold nanoparticles, NoNP no nanoparticles, HNCC head and neck cancer cells, STB(R) standard target beam (radiation), DTB(R) diamond target beam (radiation). |
|
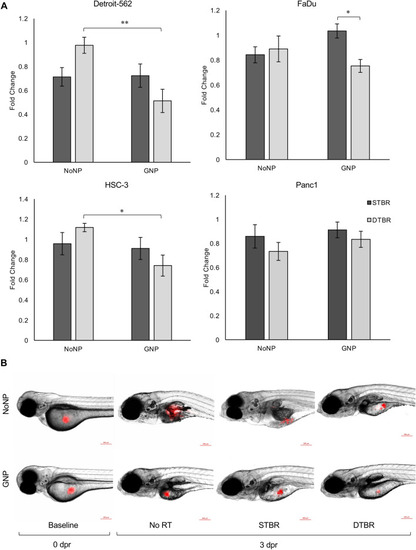
HNCCs labelled with GNPs show decreased in vitro colony forming potential 10 days post radiation. A colony formation assay (CFA) was used to determine the potential of GNP-labelled or unlabelled (NoNP) cancer cells to form colonies 10 days after radiation with the STB or DTB. ( |
|
GNP-mediated DTBR significantly decreased HNCC proliferation in vivo. Groups of 15–20 zebrafish larvae were used for each time point and treatment group, 50–100 cells were injected into the yolk sac of each fish, and the number of fluorescent cells was quantified ex vivo. Xenografted cells were quantified at baseline (0 days post radiation (dpr)) and 3 dpr after treatment with 8 Gy from the STB or DTB. Results are represented as fold change of the unirradiated control cells (No RT). ( |
|
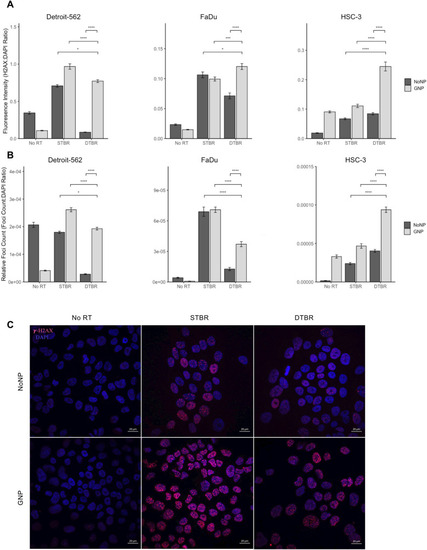
GNP labelled Detroit-562 and HSC-3 HNCCs demonstrate increased fluorescence intensity (FI) of double stranded breaks (DSBs) with DTBR compared to NoNP cells with STBR. Cells were cultured in 6-well plates, irradiated with 8 Gy from the STB or DTB, then fixed 30 min after radiation. Cells were processed for immunohistochemistry (IHC) with γ-H2AX to assess DNA double strand breaks and DAPI nuclear stain. ( |
|
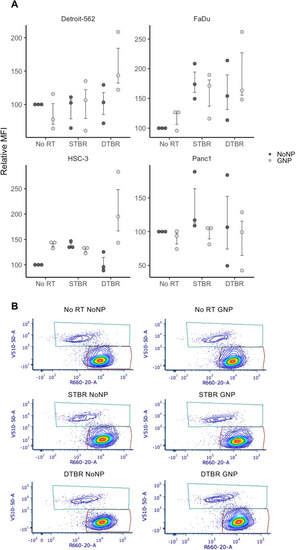
Reactive oxygen species (ROS) were elevated in HNCCs with GNP-facilitated DTBR. Cells were cultured in 6-well plates, irradiated with 8 Gy from the STB or DTB, then processed for flow cytometry 12 h post radiation. ( |