- Title
-
The zebrafish embryo as an in vivo model for screening nanoparticle-formulated lipophilic anti-tuberculosis compounds
- Authors
- Dal, N.K., Speth, M., Johann, K., Barz, M., Beauvineau, C., Wohlmann, J., Fenaroli, F., Gicquel, B., Griffiths, G., Alonso-Rodriguez, N.
- Source
- Full text @ Dis. Model. Mech.
|
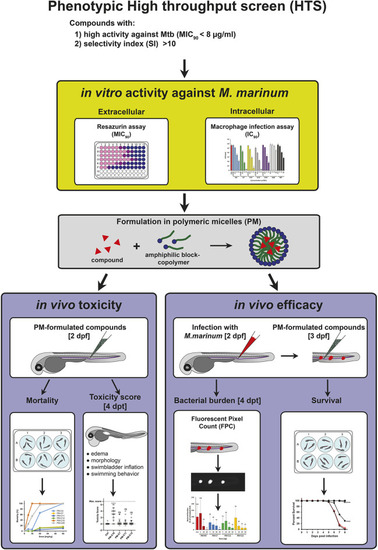
Schematic summary of the strategy used to evaluate the in vivo toxicity and anti-mycobacterial efficacy of HTS-identified lipophilic compounds using the zebrafish embryo model. Candidate compounds with significant anti-mycobacterial activity were identified in a phenotypic HTS campaign using chemical libraries and selected for further evaluation based on their in vitro activity against Mtb (MIC90), and their selectivity index (SI). Prior to in vivo testing in the zebrafish TB model using the fish pathogen Mm, candidate compounds were tested for their in vitro activity against extracellular Mm using a resazurin assay and against intracellular bacteria in mouse macrophages using a CFU assay. Then, candidate compounds were formulated in PMs, primarily to enhance solubility and to enable intravenous injection in the zebrafish embryo. The in vivo toxicity of PM-formulated compounds was first evaluated by mortality analysis after a single injection of different doses at 8 days post treatment (dpt), and compounds with significant toxicity were excluded from further analysis. PM-formulated compounds showing low mortality were then characterized in more detail by assigning a toxicity score to individual embryos at 4 dpt based on different morphological and physiological indicators of toxicity, as outlined in Materials and Methods. For the evaluation of in vivo efficacy, zebrafish embryos were infected with Mm and treated with different doses of PM-formulated compounds. Therapeutic efficacy was then assessed by bacterial burden (FPC) at 4 dpt and by survival analysis. dpf, days post fertilization. |
|
In vitro anti-mycobacterial activity of nNF derivatives against extracellular and intracellular Mm. (A) The nitroaromatic backbone structure of the nNF compounds tested in this study. (B) Activity of nNF derivatives against extracellular Mm determined by MIC90. (C) Anti-mycobacterial activity of nNF derivatives and RIF against intracellular Mm in murine BMDMs. BMDM cultures were infected with Mm at 32.5°C at a MOI of 0.1 and treated with nNF derivatives at different concentrations based on their MIC90. Intracellular mycobacterial burden was determined by CFU plating at 4 days post infection. Data represent the mean±s.d. of three independent experiments. nd, not detected. |
|
Evaluation of the in vivo toxicity of PM-formulated nNF derivatives in zebrafish embryos by mortality analysis. Zebrafish embryos were treated with different doses of PM-formulated nNF derivatives by intravenous injection, and mortality was recorded at 8 dpt. Results are pooled from at least three independent experiments. |
|
Evaluation of the in vivo toxicity of PM-formulated nNF derivatives in zebrafish embryos by toxicity score. Zebrafish embryos were injected with high doses (50 mg/kg) of PM-formulated C7, C11 and C12, or 5 mg/kg of PM-C16, and the toxicity score (TS) was determined at 4 dpt in individual embryos based on morphological and physiological indicators of toxicity, as specified in Table 1. (A) Representative images of embryos with different toxicity scores displaying normally inflated (arrowhead) or lack of inflated swim bladder (*), severe edemas (ed) and abnormally curved body shape (bottom image). Scale bar: 500 μm. (B) Toxicity scores in embryos injected with PM-formulated compounds. Non-injected (ctrl) and mock (PBS)-injected embryos were used as negative toxicity controls, and embryos injected with PM-C16 as a positive toxicity control. Each symbol represents the toxicity score of an individual larvae and the mean for each group is shown as a horizontal line with error bars denoting the s.d. Ctrl, n=18; Mock-inj., n=21; C16, n=18; C7, n=31; C11, n=26; C12, n=25); ***P<0.001 compared with the non-injected control (ctrl); ns, not significant (non-parametric Kruskal–Wallis H test followed by post-hoc analysis using Dunn's multiple comparisons test). |
|
Quantification of bacterial burden in Mm-infected zebrafish embryos after treatment with PM-formulated nNF derivatives by three different methods. Mm-dsRed-infected zebrafish embryos were treated with the indicated doses of the PM-formulated nNF derivates C7, C11, C12 or PM-formulated RIF by intravenous injection, and bacterial burden was quantified at 4 dpt by three different assays. (A) FPC: untreated or treated embryos were imaged and the total bacterial fluorescence intensity was determined by FPC for individual embryos. Data are mean±s.d. of data normalized to the untreated control (ctrl) pooled from at least three independent experiments (Ctrl, n=71; PM-C7 5 mg/kg, n=13, and 10 mg/kg, n=15; PM-C11 10 mg/kg, n=15, and 20 mg/kg, n=18; PM-C12 10 mg/kg, n=45, and 20 mg/kg, n=49; and PM-RIF 20 mg/ml, n=51). **P<0.01, ***P<0.001 (non-parametric Kruskal–Wallis H test followed by post-hoc analysis using Dunn's multiple comparisons test). (B) Plate reader (PR) assay: embryos in each treatment group or the untreated control were pooled and homogenized, and fluorescence intensity was measured using a plate reader. (C) CFU count: bacterial burden was enumerated by plating serial dilutions of the pooled homogenate on 7H10 plates. Results in B and C are shown as a percentage relative to the untreated control and represent data from one experiment. The mean values of each group in A-C are displayed above the columns. (D-F) Correlation between the normalized bacterial burden for all four compounds obtained by the three methods. Linear regression plots comparing FPC with the PR assay (D), CFU with the PR assay (E) and CFU with the FPC assay (F). Pearson's coefficient of correlation (r) with P-value are denoted in the individual figures. |
|
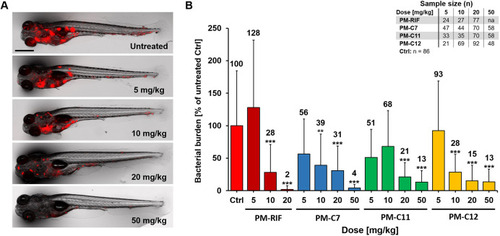
Evaluation of the in vivo efficacy of PM-formulated nNF derivatives in the zebrafish TB model. Zebrafish embryos were infected with Mm-dsRed and treated with different doses of the PM-formulated nNF derivates C7, C11, C12 or PM-formulated RIF by intravenous injection. (A) Representative images of Mm-infected embryos treated with different doses of PM-C7 at 4 dpt. Scale bar: 500 μm. (B) Quantification of bacterial burden by FPC at 4 dpt in embryos treated with the indicated doses of the PM-formulated compounds or RIF. FPC values of individual embryos were normalized to the untreated control (ctrl) group and results are presented as mean±s.d. Results are pooled from at least three independent experiments. The mean values of each group are displayed above the columns. na, not applicable. **P<0.01, ***P<0.001 (non-parametric Kruskal–Wallis H test followed by post-hoc analysis using Dunn's multiple comparisons test). |
|
In vivo efficacy of PM-formulated nNF derivatives against Mm infection in zebrafish embryos assessed by survival analysis. Mm-dsRed-infected zebrafish embryos were treated 1 day post infection with the indicated doses of the PM-formulated nNF derivates C7 (A), C11 (B), C12 (C) (5-50 mg/kg) or PM-formulated RIF (D) (5-20 mg/kg) by intravenous injection, and survival was recorded daily. Uninfected untreated embryos were used as a negative control (ctrl) and Mm-infected untreated embryos as an infection control group. Embryos used in this assay are the same as in the FPC assay (Fig. 6). Results are pooled from at least three independent experiments. na, not applicable. **P<0.01, ***P<0.001 (log-rank test of the Kaplan–Meier estimate of survival). PHENOTYPE:
|