- Title
-
Discovery of a subtype-selective, covalent inhibitor against palmitoylation pocket of TEAD3
- Authors
- Lu, T., Li, Y., Lu, W., Spitters, T., Fang, X., Wang, J., Cai, S., Gao, J., Zhou, Y., Duan, Z., Xiong, H., Liu, L., Li, Q., Jiang, H., Chen, K., Zhou, H., Lin, H., Feng, H., Zhou, B., Antos, C.L., Luo, C.
- Source
- Full text @ Acta Pharm Sin B
|
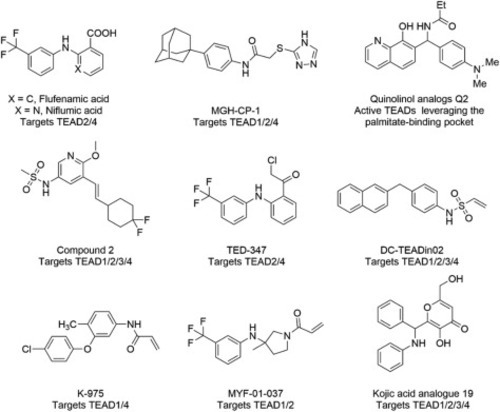
Figure 1. Reported small molecules targeting the palmitate-binding pocket of TEADs. |
|
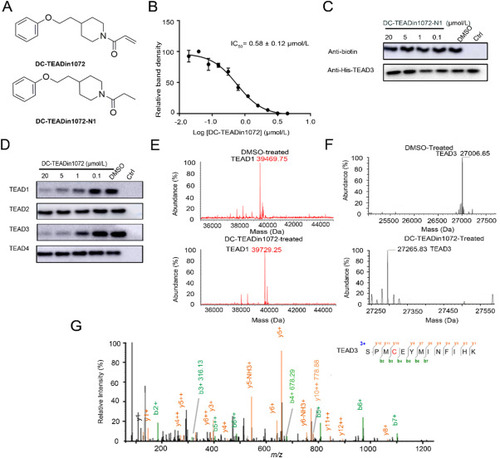
Figure 2. The discovery of DC-TEADin1072 as a covalent TEAD1 and TEAD3 autopalmitoylation inhibitor. (A) The chemical structures of DC-TEADin1072 and DC-TEADin1072-N1. (B) and (C) The inhibitory activities of DC-TEADin1072 and DC-TEADin1072-N1 in the click-based ABPP assay. The band intensity was quantified in ImageJ (NIH). The experiments were performed in biological triplicates and the data was shown as mean ± SD, n = 3. (D) DC-TEADin1072 selectively inhibited TEAD1 and TEAD3 palmitoylation while sparing TEAD2 and TEAD4. (E)‒(G) Mass spectrometry demonstrated the covalent modification of DC-TEADin1072 on TEAD1 and TEAD3 recombinant protein. |
|
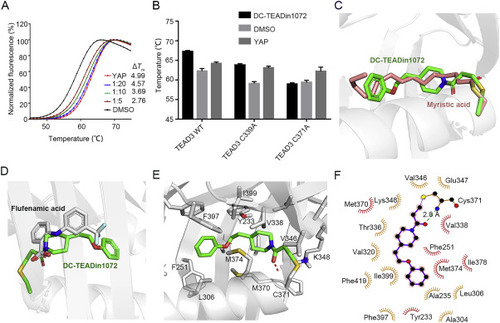
Figure 3. Biochemical and biophysical methods demonstrated the direct binding of DC-TEADin1072. (A) TEAD3 thermal shift assays. Synthetic YAP peptide was used as the positive control with the ratio of 1:5. The experiments were performed in biological triplicates. (B) The shift of Tm values of TEAD3-YBD WT and TEAD3-YBD mutants with DC-TEADin1072. (C) and (D) The alignment of DC-TEADin1072 (green) with myristic acid (salmon) (PDB code: 5OAQ)38 and flufenamic acid (grey) (PDB code: 5DQ8)33. (E) and (F) The predicted binding mode of DC-TEADin1072 with TEAD3-YBD in molecular docking studies. |
|
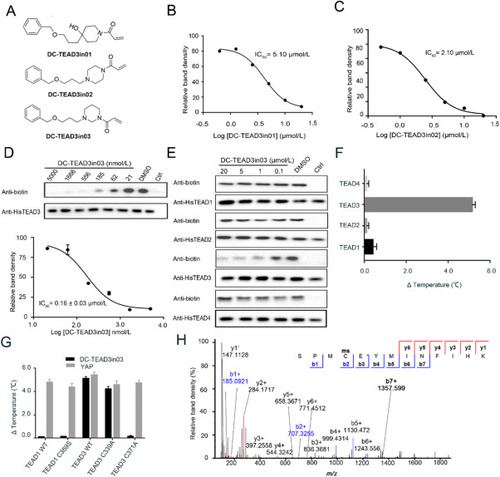
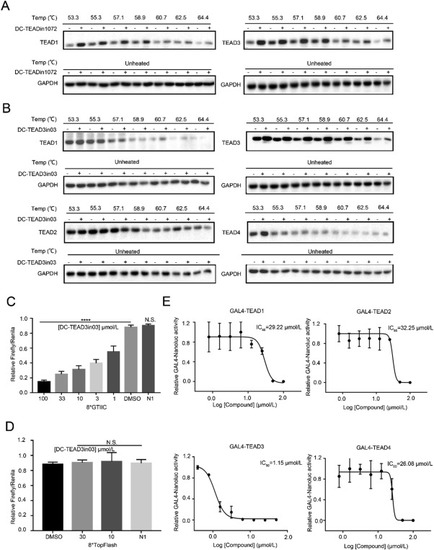
Figure 4. The biophysical and biochemical characterization of DC-TEAD3in03. (A) The chemical structures of DC-TEAD3in01, DC-TEAD3in02 and DC-TEAD3in03. (B)‒(D) The inhibitory activities of DC-TEAD3in01, DC-TEAD3in02 and DC-TEAD3in03 through CuAAC-based click chemistry. The band intensity was quantified in ImageJ (NIH). The experiments were performed in triplicate and the data was shown as mean ± SD, n = 3. (E) DC-TEAD3in03 selectively inhibited TEAD1 and TEAD3 palmitoylation while sparing TEAD2 and TEAD4. (F) and (G) DC-TEAD3in03 significantly improved the thermostability of TEAD3 but had minimal effect on TEAD1 or corresponding C359S mutant. Synthetic YAP peptide was used as the positive control with the ratio of 1:5. The experiments were performed in triplicate. (H) The modification of transfected-TEAD3 upon 50 μmol/L DC-TEAD3in03 treatment for 6 h was detected by MS experiments. |
|
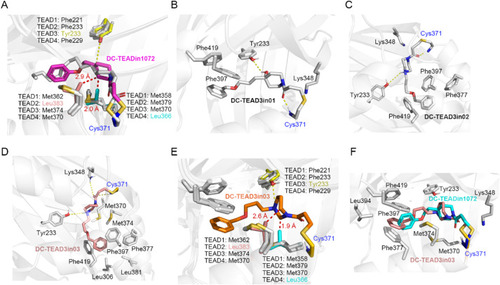
Figure 5. The binding mode analysis of DC-TEAD3in03. (A) Binding mode of DC-TEADin1072 (magenta) with TEADs protein (TEAD1: 6IM539, TEAD2: 5EMV30, TEAD3: 5EMW30, TEAD4: 3JUA40). DC-TEADin1072 forms clash (red dash) with Leu383 in TEAD2 and Leu366 in TEAD4, rationalizing the selectivity towards TEAD1 and TEAD3. (B) Binding mode of DC-TEAD3in01 with TEAD3. (C) Binding mode of DC-TEAD3in02 with TEAD3. (D) Binding mode of DC-TEAD3in03 with TEAD3. (E) The clash (red dash) between DC-TEAD3in03 (orange) and TEAD2, TEAD4. (F) Overlap mode of DC-TEADin1072 (cyan) and DC-TEAD3in03 (salmon) with TEAD3. |
|
Figure 6. DC-TEAD3in03 selectively engaged TEAD3 in cells. (A) HEK293T cells were treated with 50 μmol/L DC-TEADin1072 for 6 h in CETSA experiments to demonstrate the direct binding of DC-TEADin1072 with TEAD1 and TEAD3 in intact cells. (B) HEK293T cells were treated with 50 μmol/L DC-TEAD3in03 for 6 h in CETSA experiments to demonstrate that DC-TEAD3in03 significantly increased the thermal stability of TEAD3 in intact cells. (C) and (D) The effect of DC-TEAD3in03 on TEAD-dependent/independent reporter system. The results shown are mean ± SD of three technical replicates (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001, N.S.: P > 0.05), n = 3. (E) The effect of DC-TEAD3in03 on TEAD1, TEAD2, TEAD3, and TEAD4 activities in GAL4 reporter system. HEK293T cells were transfected with a GAL4-LUC reporter, together with expression vectors for GAL4-TEADs constructs as indicated. The results shown are mean of three technical replicates, n = 3. |
|
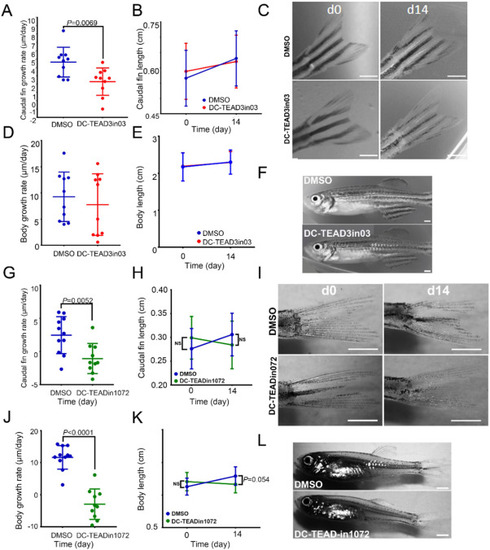
Figure 7. DC-TEAD3in03 decreases vertebrate appendage growth during the juvenile growth phase. (A) Growth rates of the caudal fins of DMSO-treated and DC-TEAD3in03-treated zebrafish during the juvenile growth phase (starting at 2 months of age). (B) The average and standard deviation of fin lengths of DMSO- and DC-TEAD3in03-treated fish at the indicated time points. (C) Caudal fins of zebrafish before treatments (d0) and after 14 days of continuous treatment (d14). (D) Growth rates of the bodies of DMSO-treated and DC-TEAD3in03-treated zebrafish during the juvenile growth phase (starting at 2 months of age). (E) Measurements of the body length of untreated and treated zebrafish during the juvenile growth phase (starting at 2 months of age). (F) Images displaying zebrafish bodies after treatment with DMSO and DC-TEAD3in03. (G) Growth rates of the caudal fins of DMSO-treated and DC-TEADin1072-treated zebrafish during the juvenile growth phase (starting at 2 months of age). (H) The average and standard deviation of fin lengths of DMSO- and DC-TEADin1072-treated fish at the indicated time points. (I) Caudal fins of zebrafish before treatments (d0) and after 14 days of continuous treatment (d14). (J) Growth rates of the bodies of DMSO-treated and DC-TEADin1072-treated zebrafish during the juvenile growth phase (starting at 2 months of age). (K) Measurements of the body length of untreated and treated zebrafish during the juvenile growth phase (starting at 2 months of age). (L) Images displaying zebrafish bodies after treatment with DMSO and DC-TEADin1072. Scale bars equal 1 mm. |