- Title
-
Notch Signaling Regulates Muscle Stem Cell Homeostasis and Regeneration in a Teleost Fish
- Authors
- Sultan, S.H.A., Dyer, C., Knight, R.D.
- Source
- Full text @ Front Cell Dev Biol
|
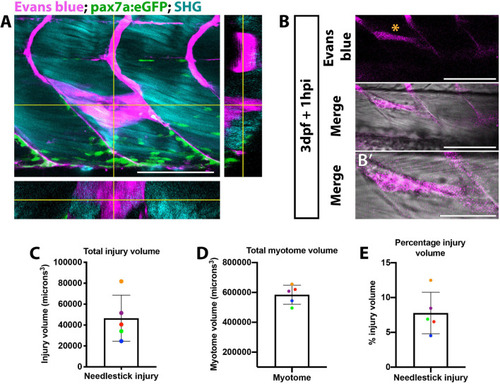
Visualization of muscle injury using Evans blue reveals low variability of injury size caused by needlestick. Evans blue labeling of damaged muscle |
|
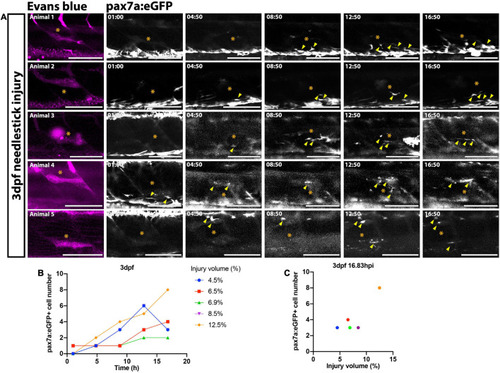
FIGURE 2. Time-lapsed imaging reveals the extent of the muSC response is related to the injury size. Images from time-lapsed movies reveal pax7a:eGFP-expressing muSCs (arrowheads) respond to injury (asterisk) labeled with Evans Blue (magenta) in the myotome of five representative 3 dpf larvae (A, animals 1–5). Images were acquired on a multiphoton microscope and maximum intensity projections generated. The number of pax7a:eGFP+ cells in the myotome was quantified and plotted against time (B) and injury volume (C). Each colored line (B) or dot (C) represents a single animal. Scale bars: 100 μm (A). |
|
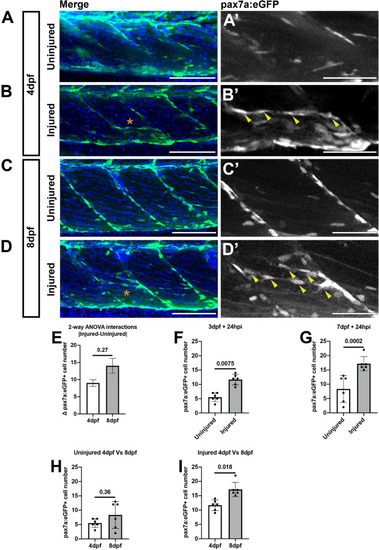
Muscle injury results in an increased number of pax7a:egfp-expressing cells within the myotome at 3 and 7 dpf. Projections of confocal z-stacks of the myotome in uninjured |
|
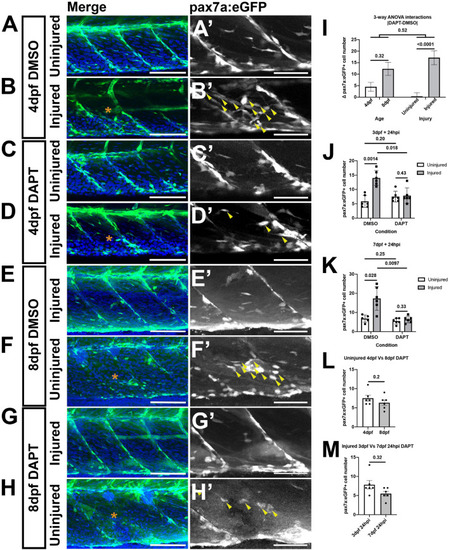
A loss of Notch activity attenuates the muSC response to injury. Projections of confocal stacks of the myotome in uninjured |
|
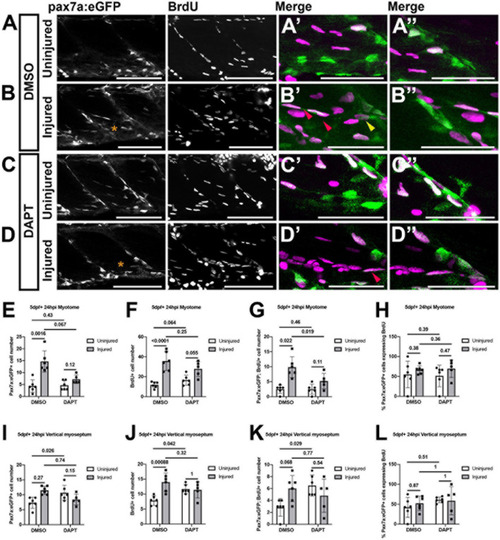
Proliferation of muSCs during regeneration and homeostasis is Notch dependent. Projections of confocal stacks |
|
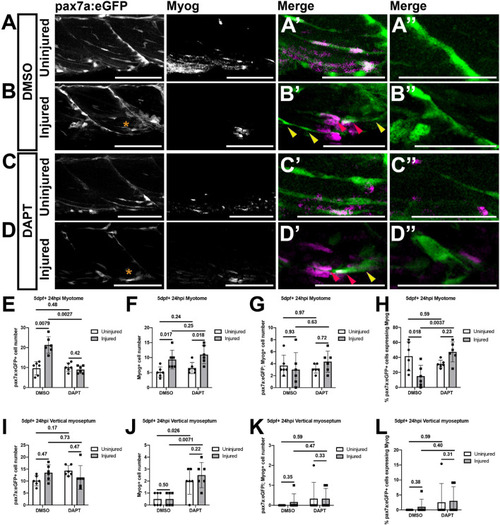
Inhibition of Notch signaling results in an increased differentiation of muSCs responding to injury. Projections of confocal stacks |