- Title
-
Increased locomotor activity via regulation of GABAergic signalling in foxp2 mutant zebrafish-implications for neurodevelopmental disorders
- Authors
- Lüffe, T.M., D'Orazio, A., Bauer, M., Gioga, Z., Schoeffler, V., Lesch, K.P., Romanos, M., Drepper, C., Lillesaar, C.
- Source
- Full text @ Transl Psychiatry
|
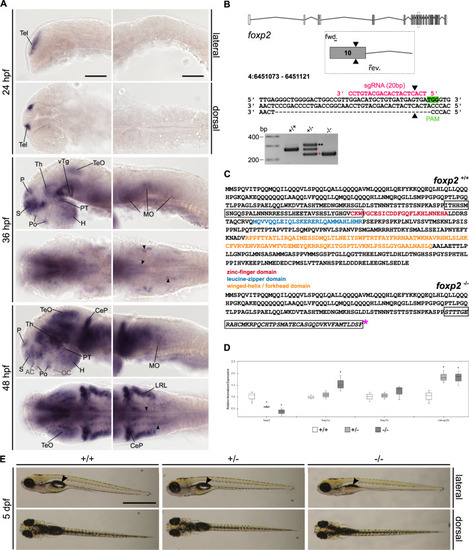
A Whole-mount RNA in situ hybridisation (ISH) in the developing zebrafish. Anterior is to the left. Abbreviations are listed in Table S4. Arrowheads indicate distinct foxp2-positive populations in the MO. Scale bars: 100 µm. B foxp2 exon–intron structure with coding (grey) and non-coding exons (white). The sgRNA (pink) targeting foxp2 exon 10 and the primer binding sites for genotyping PCR are indicated. The CRISPR/Cas9-induced double-strand break (black triangle) caused a 40 bp deletion represented by a PCR product of 228 bp (pink asterisk) in foxp2+/− and foxp2−/−. A third band of ~320 bp corresponds a heterodimer of wildtype and mutated PCR product (black asterisks). C Predicted amino acid sequence of the wildtype (top) and mutated allele (bottom). The frameshifted sequence (black box) is interrupted by a premature stop codon (pink asterisk) N-terminal of the zinc-finger domain. D Relative normalised expression of foxp2, foxp1a, foxp1b and cntnap2b in foxp2+/+ (white), foxp2+/− (light grey) and foxp2−/− (dark grey) based on qPCR. *P < 0.05. E Live images of 5 dpf old foxp2+/+, foxp2+/− and foxp2−/−. Anterior is to the left. foxp2−/− show alterations in the development/inflation of the swim bladder (black arrow) in 20% of the cases. Scale bar, 1 mm. |
|
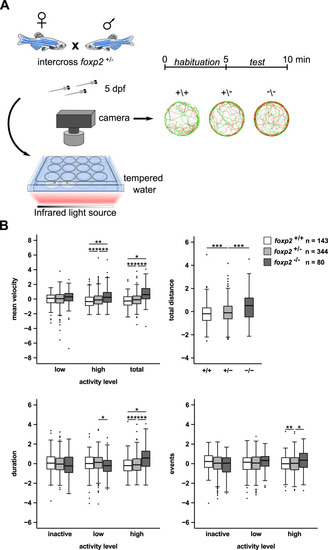
A Crossing scheme and behavioural setup applied for locomotor tracking in 5 dpf old foxp2+/+, foxp2+/− and foxp2−/−. Circles illustrate representative swim tracks of individual fish with inactive (<0.2 cm/s, black), low activity (>0.2 cm/s and <1 cm/s, green) and high activity (>1 cm/s, red). B Locomotor activity of foxp2+/+ (white), foxp2+/− (light grey) and foxp2−/− (dark grey) analysed for mean velocity (top left) in low or high activity, or combined (total), total distance swum (top right), duration (bottom left) and events (bottom right) of inactivity, low and high activity. Raw data was standardised using z-score transformation. *P < 0.05, **P < 0.01, ***P < 0.001. PHENOTYPE:
|
|
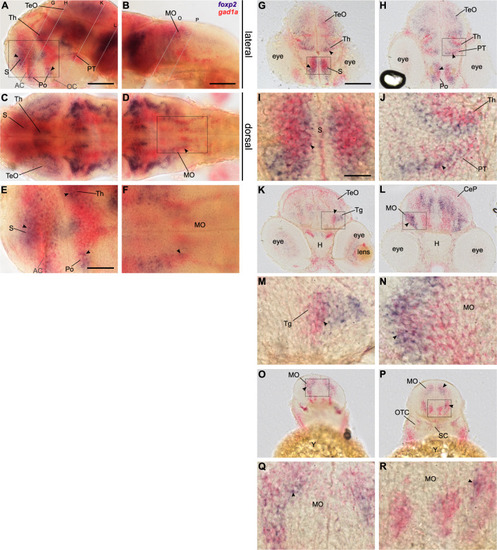
Double labelling of foxp2 (blue) and gad1a (red) expression in 48 hpf old wildtype embryos using two-colour RNA ISH. Lateral (A, B, E) and dorsal (C, D, F) overview of embryonic CNS with anterior to the left. E, F Magnifications of boxed areas in A and D. Dashed lines indicate cutting sites for cross-sections displayed in G–R. I, J, M, N, Q, R magnifications of boxed areas in G, H, K, L, O, P, respectively. Arrows indicate sites of co-localisation. Abbreviations are listed in Table S4. Scale bars: 100 µm (overview), 50 µm (magnified images). EXPRESSION / LABELING:
|
|
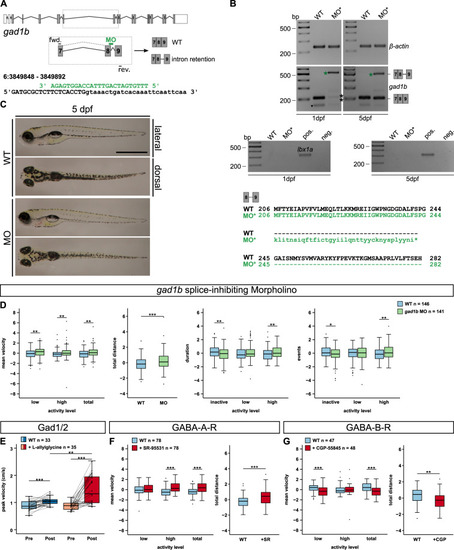
A gad1b exon–intron structure with coding (grey) and non-coding exons (white). gad1b splice-inhibiting morpholino (MO, green) and corresponding primer binding sites are indicated. Binding of the gad1b-MO causes retention of intron 8. B, top and centre The reduction of the gad1b wildtype transcript at 1 dpf (black asterisks, 208 bp) and the presence of the misspliced transcript at 1 and 5 dpf (green asterisk, 500 bp) was verified by RT-PCR. A primer off-target (black triangle) was detected below the wildtype gad1b transcript. Control PCRs on beta-actin (above, 239 bp) and lbx1a (below, 353 bp) confirmed comparable cDNA levels and absent genomic DNA contamination for all samples. B, bottom The predicted amino acid sequence of the misspliced gad1b transcript suggests a premature termination of translation in gad1b intron 8 (asterisk). C Overviews of a 5 dpf old gad1b morphant (MO) and an uninjected control larva (WT). Scale bar, 1 mm. D Locomotor activity of 5 dpf old gad1b morphant larvae (MO, green) compared to uninjected controls (WT, light blue). Locomotor activity was assessed by mean velocity in low or high activity, or combined (total), total distance swum, duration or events of inactivity, low and high activity. E Maximum mean velocity (of 5 min) reached within 8 h of tracking (peak velocity in cm/s) after incubation (Post) in 100 mM Gad-inhibitor L-allylglycine (dark red) or Danieau’s solution (control, dark blue). Maximum mean velocity (of 5 min) of the same individuals before incubation in L-allylglycine (Pre, light red) or Danieau’s solution (Pre, light blue). Line plot displays activity changes of individual larvae. F Locomotor activity of 5 dpf old wildtype larvae injected with 10 mM GABA-A-receptor antagonist SR-95531 (red) or water (light blue) at one-cell stage. G Effect of 0.1 mM GABA-B-receptor antagonist CGP-55845 on locomotor activity of 5 dpf old wildtype larvae (red) in comparison to Danieau’s solution incubated controls (light blue). D, F, G Raw data was standardised using z-score transformation. *P < 0.05, **P < 0.01, ***P < 0.001. PHENOTYPE:
|
|
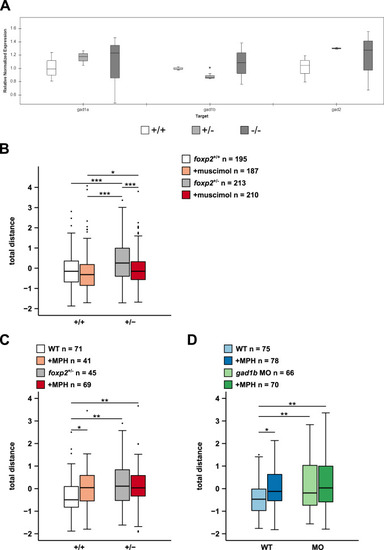
A Transcript levels of gad1a, gad1b and gad2 in foxp2+/+ (white), foxp2+/− (light grey) and foxp2−/− (dark grey) siblings assessed by qPCR. *P < 0.05. B Locomotor activity, displayed as total distance swum, of 5 dpf old foxp2+/+ and foxp2+/− following a bath-application in 0.05 mM GABA-A-R agonist muscimol (light red and dark red, respectively) or Danieau’s solution (white and grey, respectively). C Locomotor activity, displayed as total distance swum, of 5 dpf old foxp2+/+ and foxp2+/− after exposure to Danieau’s solution (white and grey, respectively) or 0.012 mM methylphenidate (MPH, light red and dark red, respectively). D Locomotor activity, displayed as total distance swum, of 5 dpf old gad1b morphants (MO) and wildtype controls (WT) exposed to Danieau’s solution (light green and light blue, respectively) or 0.012 mM MPH (dark green and dark blue, respectively). Raw data was standardised using z-score transformation. *P < 0.05, **P < 0.01, ***P < 0.001. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|