- Title
-
Yap Regulates Müller Glia Reprogramming in Damaged Zebrafish Retinas
- Authors
- Lourenço, R., Brandão, A.S., Borbinha, J., Gorgulho, R., Jacinto, A.
- Source
- Full text @ Front Cell Dev Biol
|
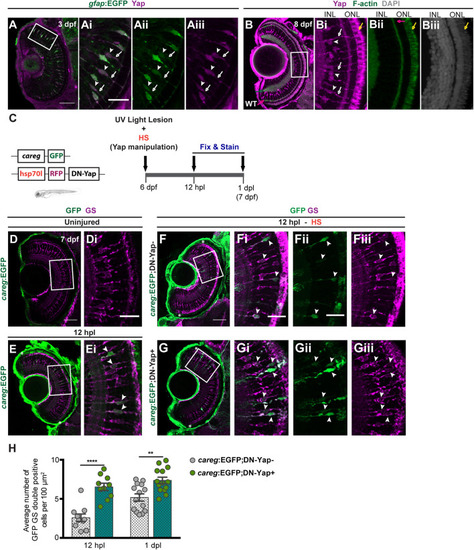
Yap inhibition induces accumulation of activated Müller glial cells (MGs) upon photoreceptor-induced light lesion. |
|
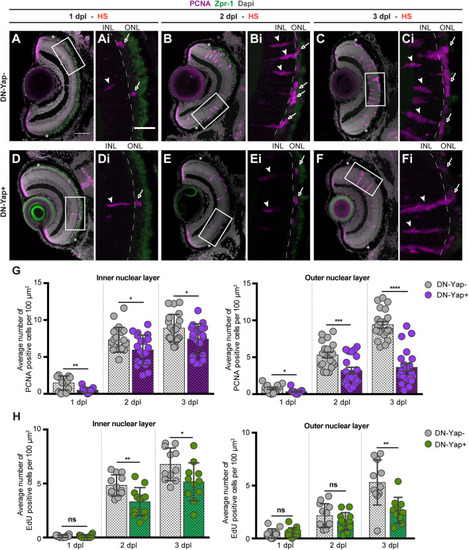
Yap inhibition reduces cell proliferation in the retina after photoreceptor-induced light lesion. |
|
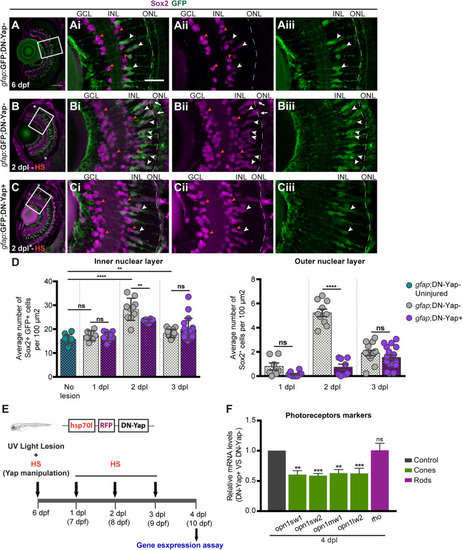
Yap is required to regulate the number of Sox2-positive Müller glia cell (MG) progenitor cells and expression of photoreceptor markers after photoreceptor-induced light lesion. |
|
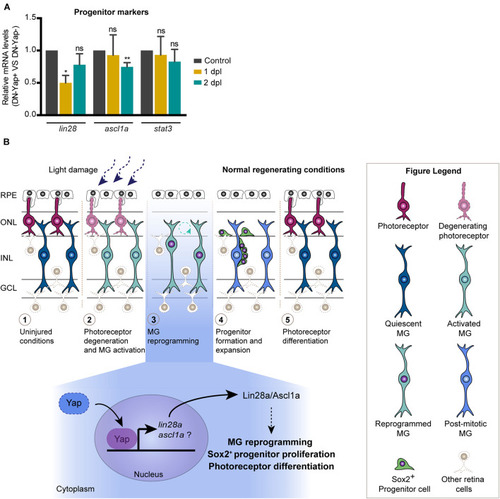
Model for the role of Yap during zebrafish retina regeneration. |