- Title
-
Collective behavior emerges from genetically controlled simple behavioral motifs in zebrafish
- Authors
- Harpaz, R., Aspiras, A.C., Chambule, S., Tseng, S., Bind, M.A., Engert, F., Fishman, M.C., Bahl, A.
- Source
- Full text @ Sci Adv
|
( |
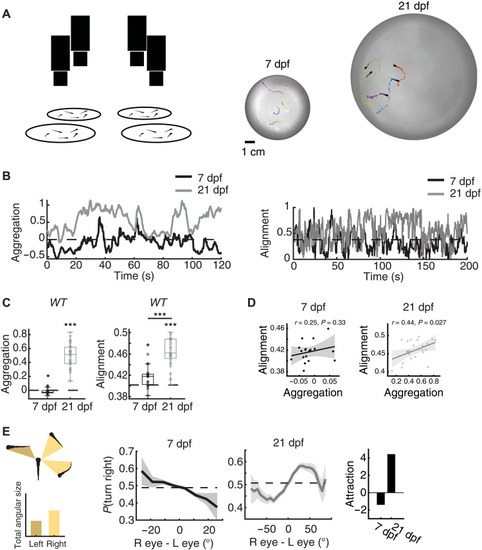
|
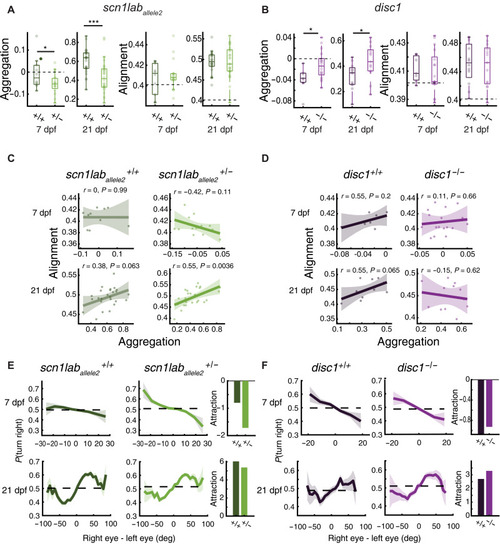
+/+, +/− , and –/– refer to sibling-controlled wild-type and mutant fish. ( |
|
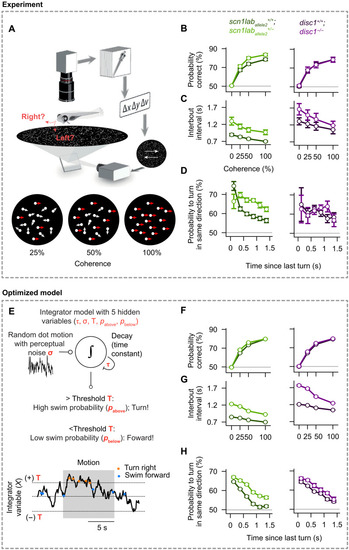
( |
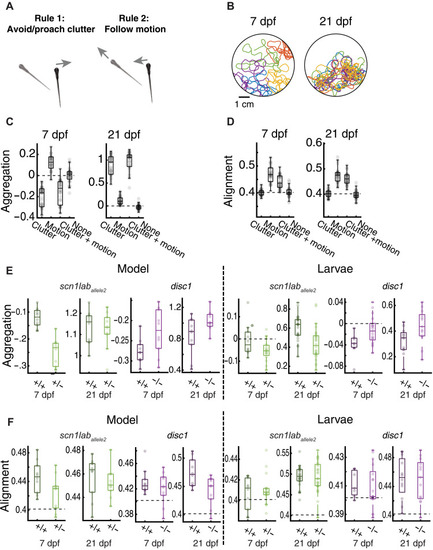
|
( |