- Title
-
Mechanosensitive Notch-Dll4 and Klf2-Wnt9 signaling pathways intersect in guiding valvulogenesis in zebrafish
- Authors
- Paolini, A., Fontana, F., Pham, V.C., Rödel, C.J., Abdelilah-Seyfried, S.
- Source
- Full text @ Cell Rep.
|
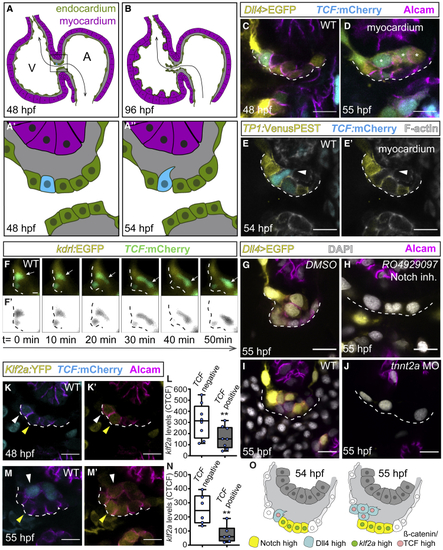
Dll4 and TCF reporters are co-expressed in ingressing endocardial cells (A–B) Model depicting the zebrafish embryonic heart. (A and A′) At 48 hpf, the superior AVC endocardium (black square in A) is monolayered and blood flow is bi-directional (black arrow) (A′). (A″) At 54 hpf, single cells ingress into the cardiac jelly. (B) At 96 hpf, valve leaflets have formed, and blood flow is uni-directional (black arrow). (C–E) Single confocal z section plane images of the superior AVC endocardium. (C and D) Single cells express (F) Light-sheet microscopy time-lapse images of a TCF-positive ingressing endocardial cell (white arrows) at the superior AVC. (F′) Black and white rendering highlights the ingressing cell. (G–J) Single confocal z section plane images of the superior AVC endocardium at 55 hpf. (G and H) Upon Notch inhibition, the superior AVC endocardium remains monolayered and has a decreased expression of the Dll4 reporter. (I and J) (K–N) Quantification of (O) Model depicting the superior AVC endocardium at stages when singled-out cells ingress into the cardiac jelly. Scale bars, 5 μm. |
|
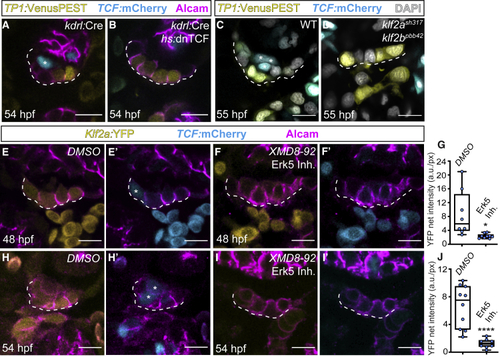
Erk5-Klf2a-Wnt signaling within AVC endocardium is essential for valvulogenesis (A–D) Single confocal z section plane images of the superior AVC endocardium at 54 hpf. (A and B) The endothelial-specific knockdown of canonical Wnt signaling prevents AVC endocardial cells from ingressing into the cardiac jelly. (A) Presence of a TCF-positive ingressed cell when TCF is not blocked (asterisk). Note the presence of TCF-positive cells in the myocardium of treated hearts (B), which confirms the tissue-specificity of this treatment. (C and D) TCF-positive endocardial cells (asterisks in C) are absent in in (E–J) Single confocal z section plane images of the superior AVC endocardium at 54 hpf. (E–G and H–J) Quantification of |
|
(A and B) Whole-mount (C–F) Single confocal z section plane images of the superior AVC endocardium at 72 hpf. (C and D) Normal atrioventricular valvulogenesis with TCF-positive cells on the abluminal side of the forming valve (asterisks) in wild-type (C) and (G and H) Single confocal z section plane images of the AVC endocardium at 96 hpf. (G) In wild type, the primordium of the AV valve leaflet has formed by 96 hpf. (H) Valvulogenesis is completely aborted and the AV endocardium remains monolayered at 96 hpf upon the MO-mediated knockdown of (I and J) Model depicting the AVC region of the zebrafish endocardium at 96 hpf. (I) Primordium of the wild-type valve leaflet (as in G). (J) AVC endocardium at 96 hpf upon loss of Wnt9a (as in H). Scale bars, 5 μm |
|
Notch-Dll4 signaling singles out cells that are competent to respond to Wnt9a (A–D) Single confocal z section plane images of the superior atrioventricular endocardium at 54 hpf (A and B) and 55 hpf (C and D). (B) Treating embryos with the Notch inhibitor (E–G) Quantification of (H) Whole-mount (I) Quantitative real-time PCR quantifications of (J and K) Single confocal z section plane images of the superior AVC endocardium at 55 hpf. (J) The ingression of endocardial cells is marked with asterisks. (K) Endocardial TCF reporter expression is lost upon MO-mediated knockdown of (L–O) Single confocal z section plane images of the superior AVC endocardium at 72 hpf. Shown are representative cell clones in Tg (P) Quantifications of ratio of clones that ingressed into the abluminal side with respect to the total number of clones integrated in the superior AVC endocardium ( (Q) Model of blood-flow-dependent molecular mechanisms that result in singling out of atrioventricular endocardial cells at the beginning of valve morphogenesis. Scale bars, 5 μm |