- Title
-
Stretching of the retinal pigment epithelium contributes to zebrafish optic cup morphogenesis
- Authors
- Moreno-Mármol, T., Ledesma-Terrón, M., Tabanera, N., Martin-Bermejo, M.J., Cardozo, M.J., Cavodeassi, F., Bovolenta, P.
- Source
- Full text @ Elife
|
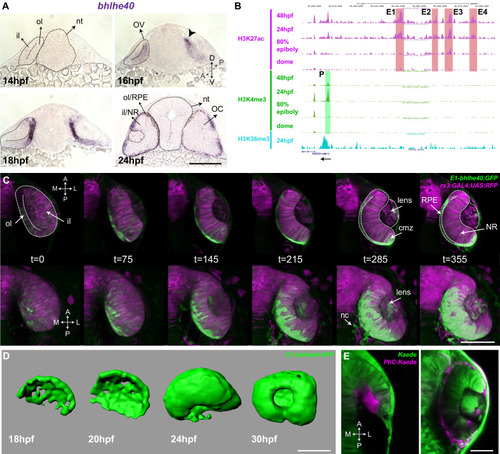
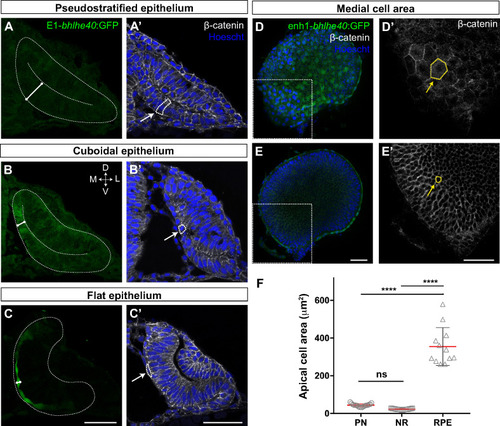
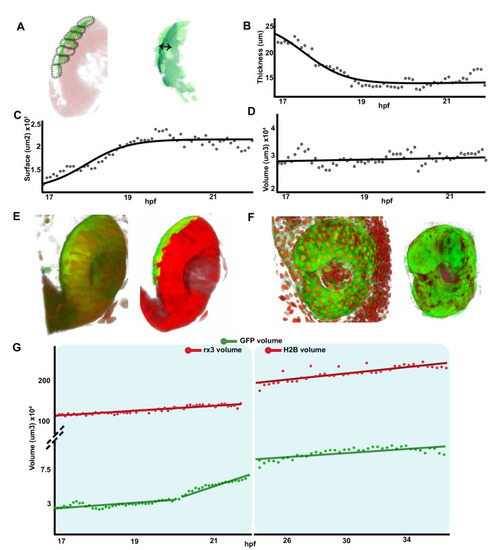
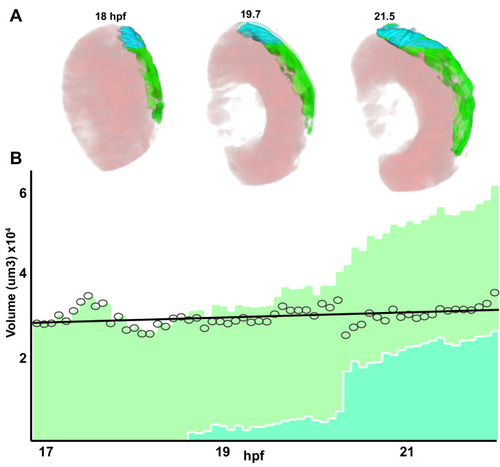
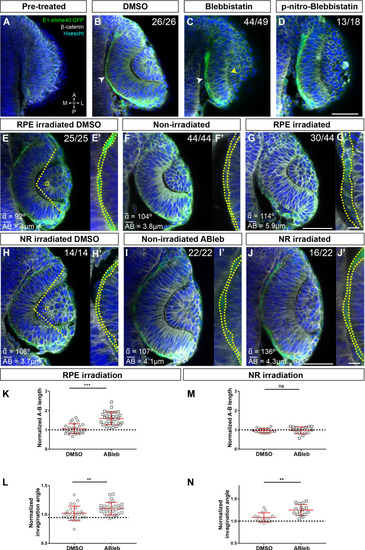
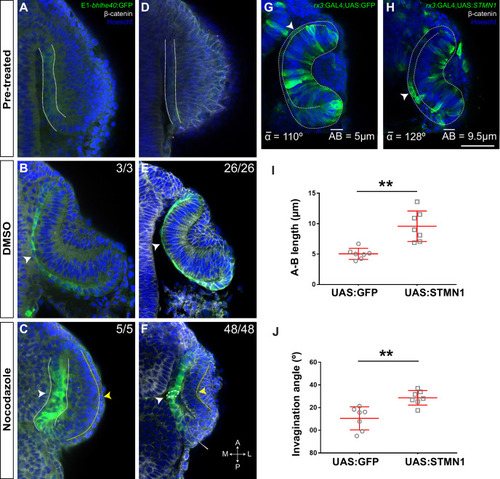
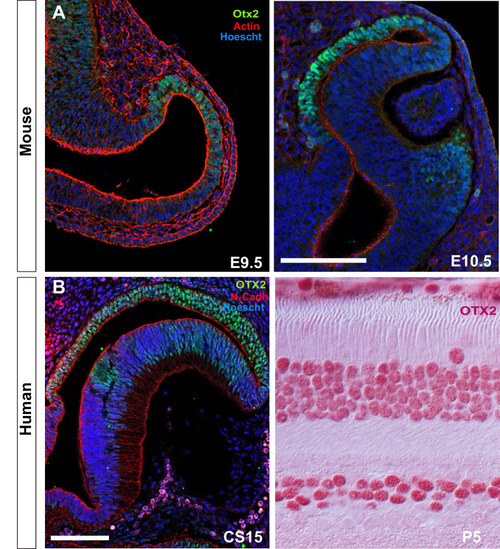
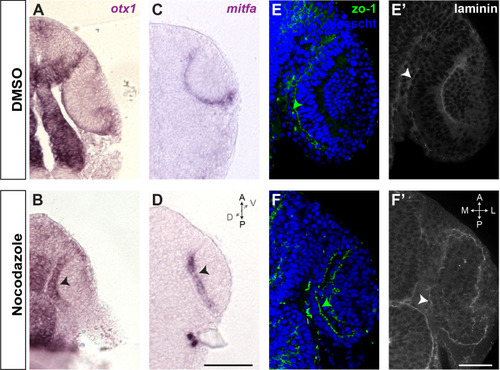
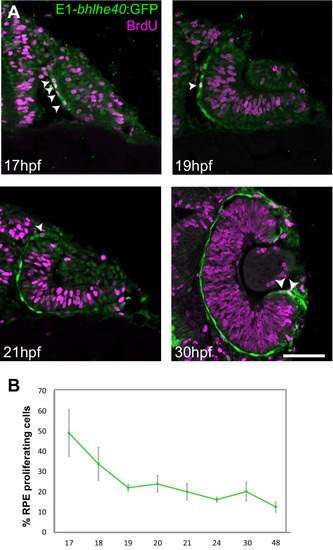
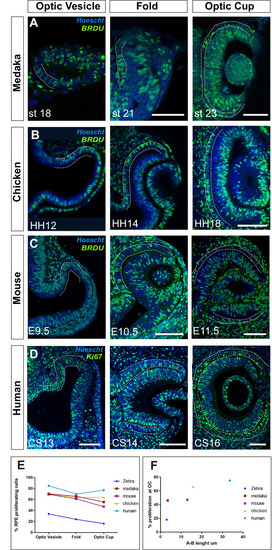
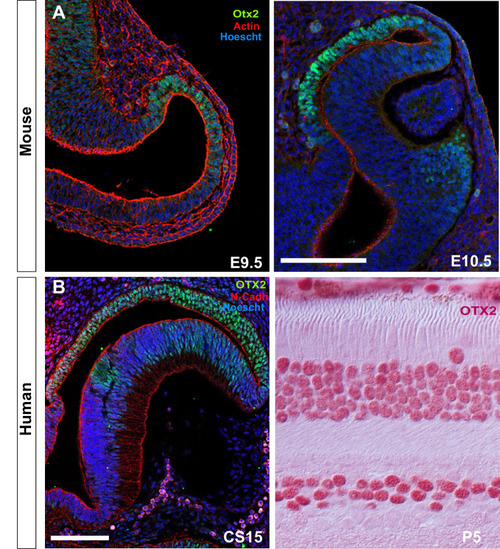
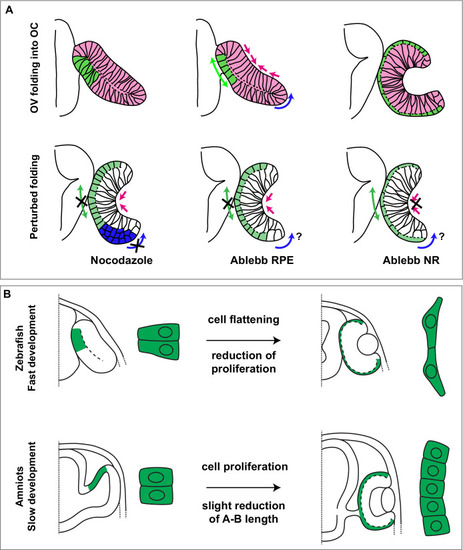
Single confocal section, related to |
|
( |
|
|
|
|
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |