- Title
-
Cholenic acid derivative UniPR1331 impairs tumor angiogenesis via blockade of VEGF/VEGFR2 in addition to Eph/ephrin
- Authors
- Rusnati, M., Paiardi, G., Tobia, C., Urbinati, C., Lodola, A., D'Ursi, P., Corrado, M., Castelli, R., Wade, R.C., Tognolini, M., Chiodelli, P.
- Source
- Full text @ Cancer Gene Ther.
|
|
|
|
|
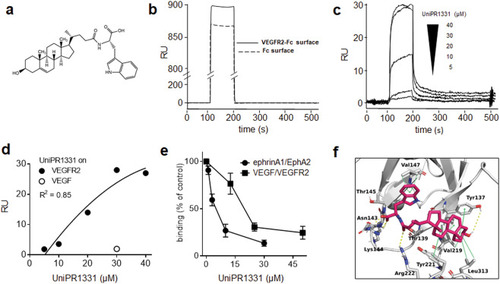
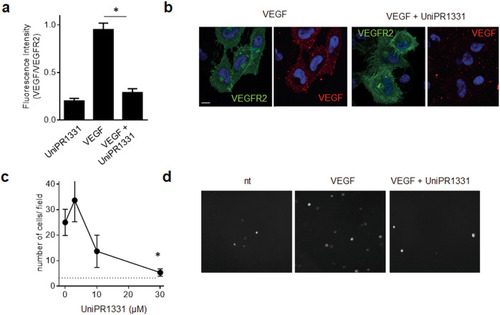
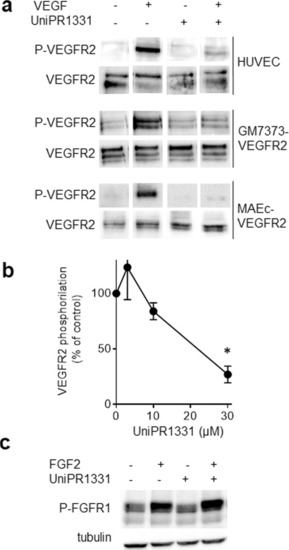
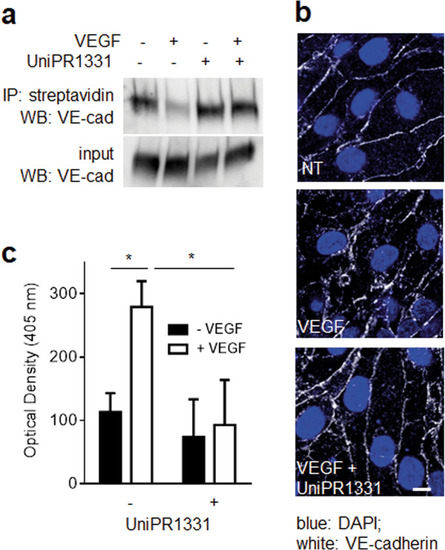
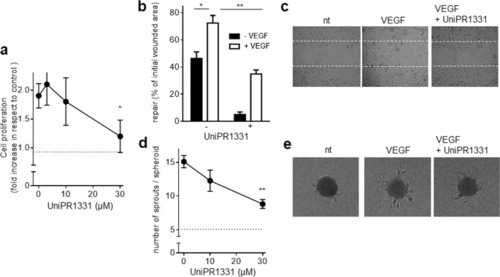
The indicated cells were left unstimulated or stimulated with 10 ng/ml VEGF ( |
|
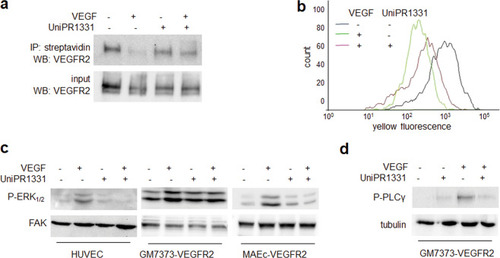
HUVECs unstimulated or stimulated with VEGF and UniPR1331 were analyzed for VEGFR2 internalization by WB ( |
|
|
|
|
|
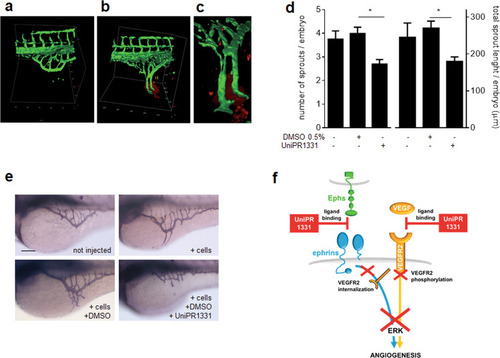
3D images reconstruction of SIV in Tg( |