- Title
-
A Novel Lysolecithin Model for Visualizing Damage in vivo in the Larval Zebrafish Spinal Cord
- Authors
- Morris, A.D., Kucenas, S.
- Source
- Full text @ Front Cell Dev Biol
|
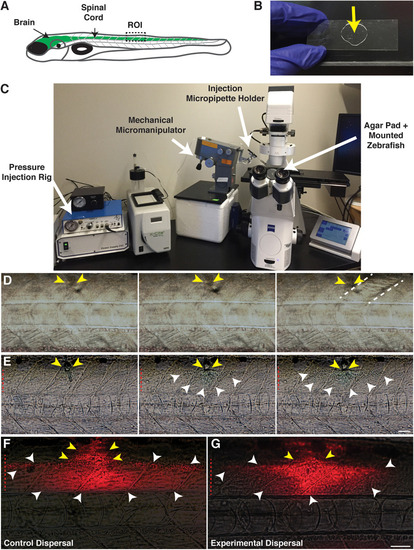
Focal delivery of solutions into the zebrafish spinal cord. |
|
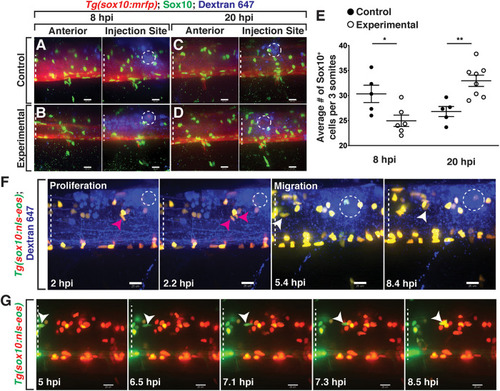
Lysolecithin alters the number and behavior of Sox10+ cells in the spinal cord. All images are lateral views of the spinal cord with anterior to the left and dorsal to the top. |
|
|
|
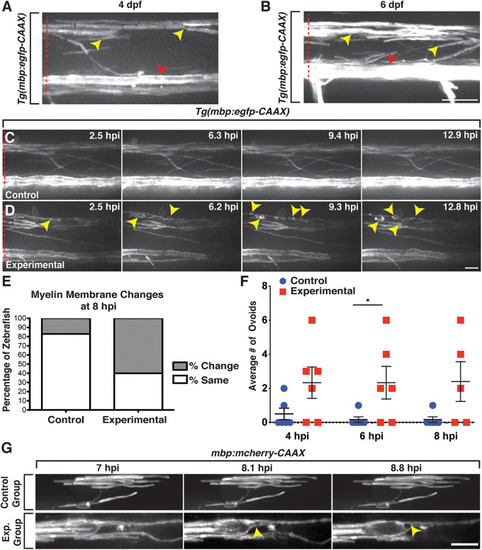
Axons are indistinguishable between control and lysolecithin-injected larvae. All images are lateral views of the spinal cord with anterior to the left and dorsal to the top. |
|
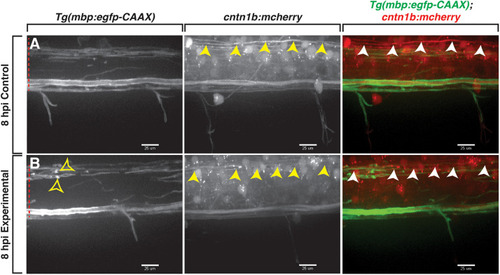
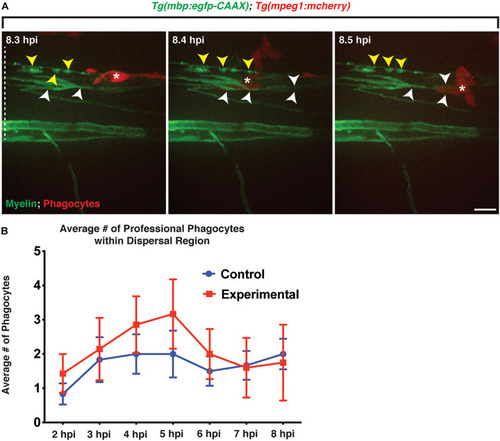
Professional phagocytes traverse through mbp+ membrane layers. All images are lateral views of the spinal cord with anterior to the left and dorsal to the top. (A) Following injection of lysolecithin, a mCherry+ phagocyte is observed traversing through mbp+ membrane layers within the lesion area, physically moving the layers during its migration. White arrowheads identify the layers that are moved by the phagocyte, and yellow arrowheads denote the myelin ovoids. (B) Quantification of the average number of professional phagocytes responding to the lesion following injection of a control (control) solution (n = 6) or lysolecithin (experimental) (n = 7); p = 0.0864. White dashed line denotes the spinal cord. Scale bars, 25 μm. |
|
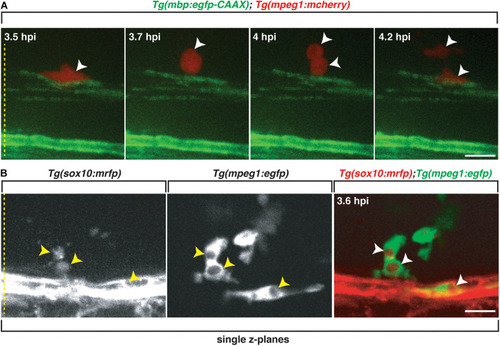
Professional phagocytes proliferate and clear Sox10+ debris. All images are lateral views of the spinal cord with anterior to the left and dorsal to the top. |
|
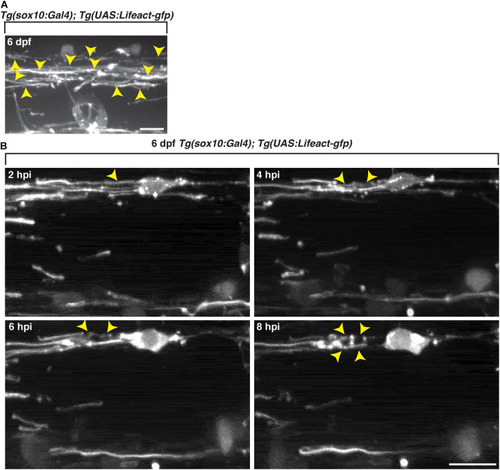
Oligodendrocyte cytoskeletal components dynamically change following exposure to lysolecithin. All images are lateral views of the spinal cord with anterior to the left and dorsal to the top. |