- Title
-
Glucocorticoid-Induced Exacerbation of Mycobacterial Infection Is Associated With a Reduced Phagocytic Capacity of Macrophages
- Authors
- Xie, Y., Xie, J., Meijer, A.H., Schaaf, M.J.M.
- Source
- Full text @ Front Immunol
|
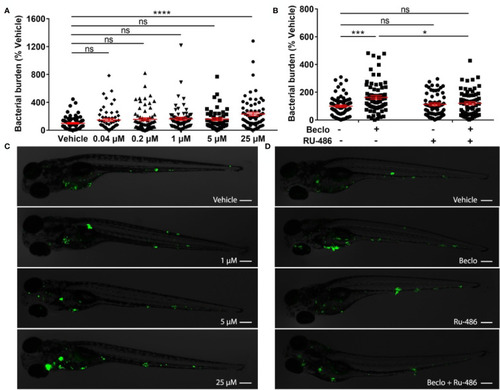
Effect of beclomethasone on PHENOTYPE:
|
|
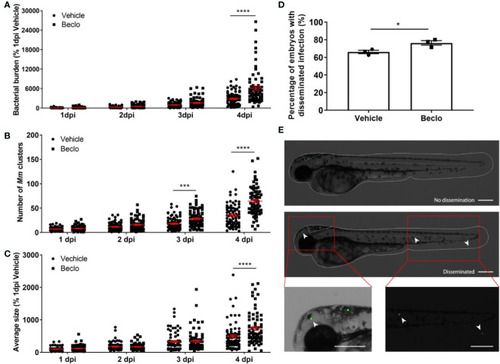
Beclomethasone effects on |
|
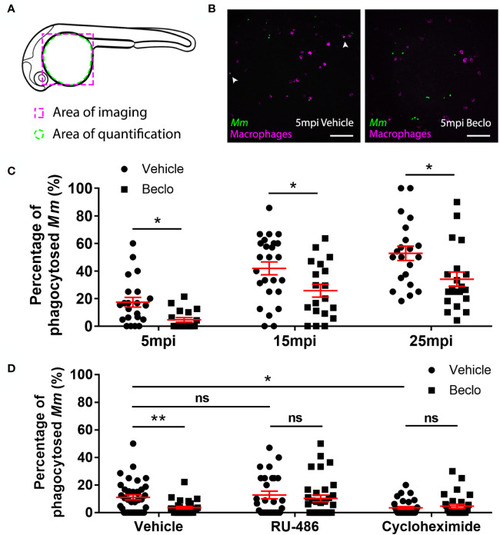
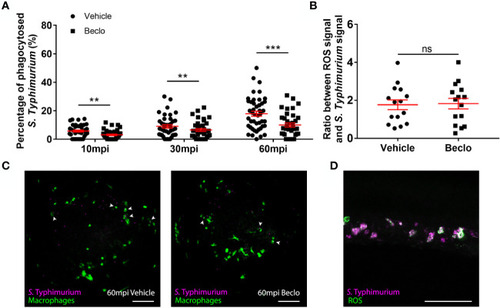
Effect of beclomethasone on phagocytic activity of macrophages and its dependency on Gr and de novo protein synthesis. (A) Schematic drawing of a zebrafish embryos at 28 hpf indicating the areas of imaging (purple dashed box, used for representative images) and quantification (green dashed circle) of Mm phagocytosis. (B) Representative confocal microscopy images of embryos of the Tg(mpeg1:mCherry-F) line injected with Mm at 28 hpf. Images were taken of infected embryos that were vehicle- or beclomethasone-treated at 5 minutes post infection (mpi). Macrophages are shown in magenta, bacteria in green. Scale bar = 100 μm. Arrowheads indicate bacterial clusters phagocytosed by macrophages. (C) Percentages of phagocytosed Mm clusters (of total number of Mm clusters) at 5, 15 and 25 mpi. Statistical analysis, performed by fitting data to a beta inflated regression with Tukey’s post hoc test, showed that beclomethasone decreased this percentage at all three time points. (D) Effects of RU-486 and cycloheximide on the beclomethasone-inhibited phagocytic activity. Embryos were treated with vehicle or beclomethasone and received either a vehicle, RU-486 or cycloheximide co-treatment two hours before injection of Mm at 28 hpf, and phagocytic activity was determined at 5 mpi. The significant inhibitory effect of beclomethasone on phagocytosis was not observed in the presence of RU-486. Cycloheximide, just like beclomethasone, significantly inhibited the phagocytic activity, and the combined cycloheximide/beclomethasone treatment showed the same level of inhibition. Statistical analysis was performed by fitting data to a beta inflated regression with Tukey’s post hoc test. In panels (C, D), each data point represents a single embryo and the means ± s.e.m. of data accumulated from three independent experiments are shown in red. Statistical significance is indicated by: ns, non-significant; *P<0.05; **P<0.01. |
|
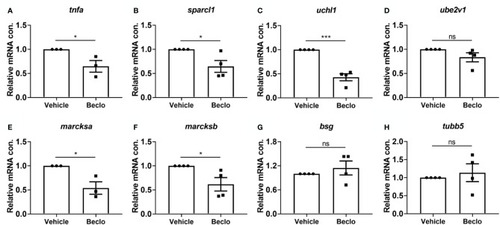
Effect of beclomethasone on gene expression levels in FACS-sorted macrophages. At 28 hpf, |
|
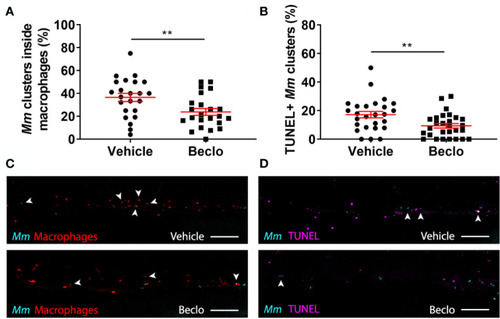
Effect of beclomethasone on intracellular bacterial growth and cell death. Infection was performed in PHENOTYPE:
|
|
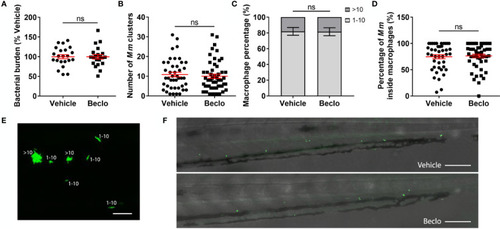
Effect of beclomethasone on the bacterial growth of the Mm Δerp mutant. The Mm Δerp mutant strain was injected intravenously at 28 hpf, and at 44 hpi, the bacterial burden (A), the number of Mm clusters (B), the percentage of macrophages that contained 1-10 or more than 10 bacteria (of all macrophages containing bacteria) (C), and the percentage of Mm inside macrophages (D) were determined. No significant difference was observed between the vehicle- and beclomethasone-treated groups for any of these parameters. Statistical analysis was performed using two-tailed t-tests. Values shown are the means ± s.e.m. of three independent experiments, with each data point representing a single embryo. Statistical significance is indicated by: ns, non-significant. (E) Representative confocal microscopy image of Mm Δerp bacterial clusters (bacteria in green), indicated are clusters containing 1-10 bacteria and clusters containing more than 10 bacteria. Scale bar = 20 μm. (F) Representative images of the tail regions of a vehicle- and a beclomethasone-treated embryo infected with Mm Δerp bacteria. Scale bar = 100 μm. |
|
Effect of beclomethasone on phagocytosis of PHENOTYPE:
|