- Title
-
Retrograde mitochondrial transport is essential for organelle distribution and health in zebrafish neurons
- Authors
- Mandal, A., Wong, H.C., Pinter, K., Mosqueda, N., Beirl, A., Lomash, R.M., Won, S., Kindt, K.S., Drerup, C.M.
- Source
- Full text @ J. Neurosci.
|
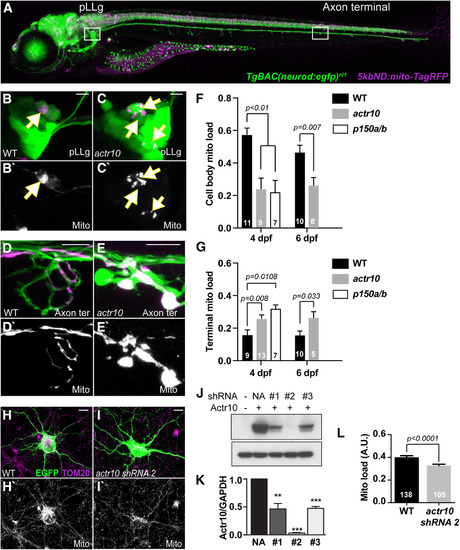
Disruption of retrograde mitochondrial movement impacts mitochondrial distribution in neurons. |
|
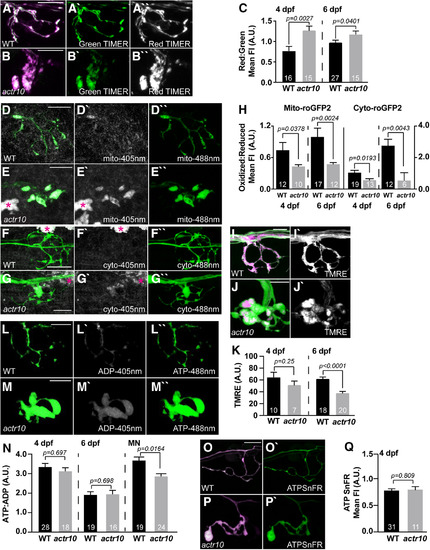
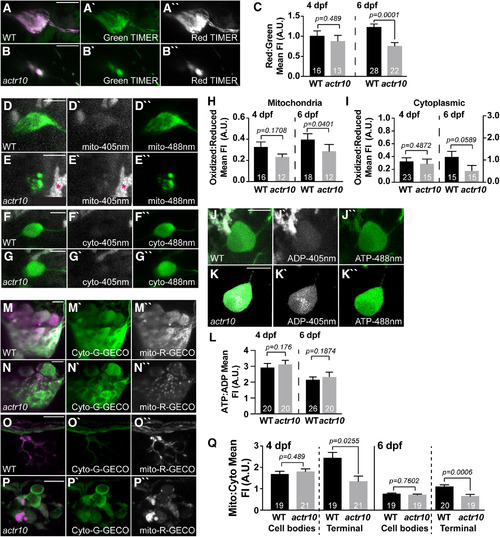
Loss of retrograde mitochondrial transport results in altered measures of mitochondrial health. |
|
Mitochondrial calcium load is decreased in axon terminal mitochondria in |
|
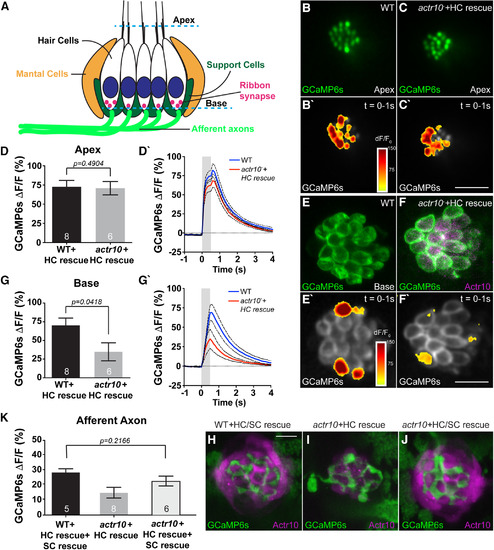
Postsynaptic axon response to stimulation is unaffected by mitochondrial health. |
|
Loss of support cells (SCs) underlies the loss of postsynaptic activity in |
|
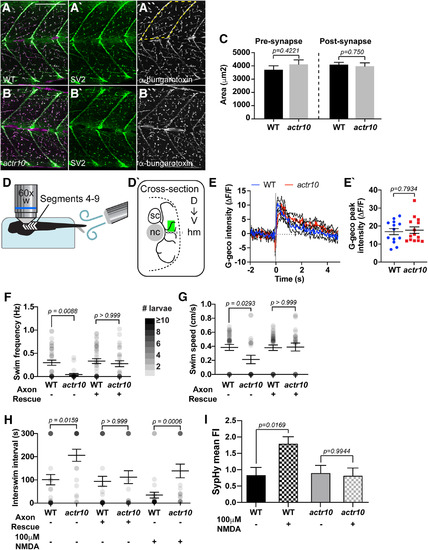
Motor neuron function is decreased in |
|
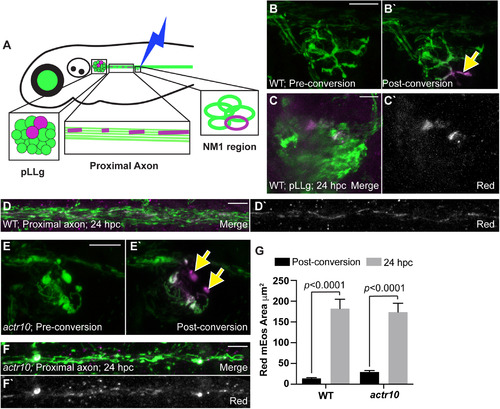
Mitochondrial retrograde transport is required for mitochondrial turnover in axon terminals. |
|
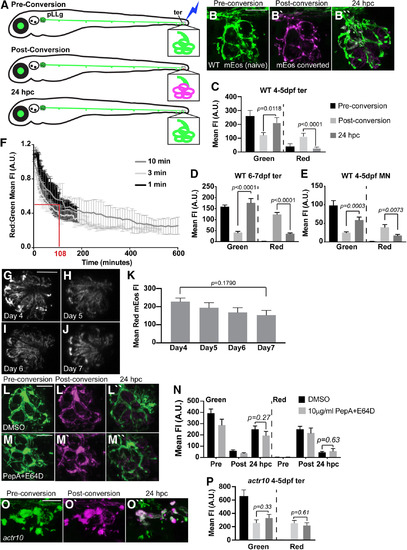
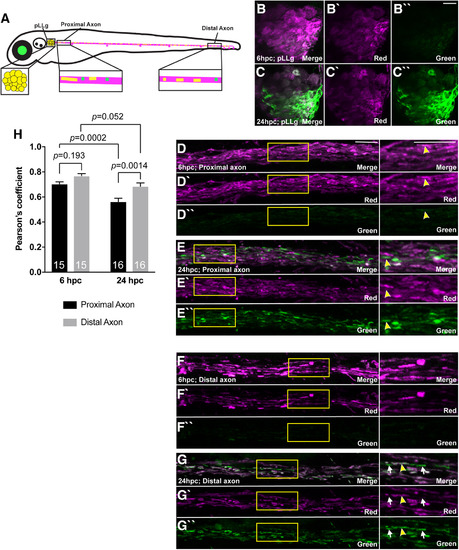
mEos can be redistributed independent of retrograde transport in pLL neurons. |
|
Mitochondrially localized mEos persists for days in neurons. |
|
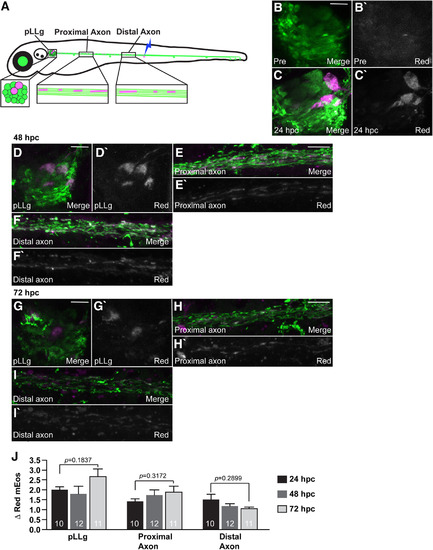
A strong correlation between red and green mEos 24 hpc suggests protein mixing within existing organelles. |