- Title
-
A Mutation in VWA1, Encoding von Willebrand Factor A Domain-Containing Protein 1, Is Associated With Hemifacial Microsomia
- Authors
- Wang, Y., Ping, L., Luan, X., Chen, Y., Fan, X., Li, L., Liu, Y., Wang, P., Zhang, S., Zhang, B., Chen, X.
- Source
- Full text @ Front Cell Dev Biol
|
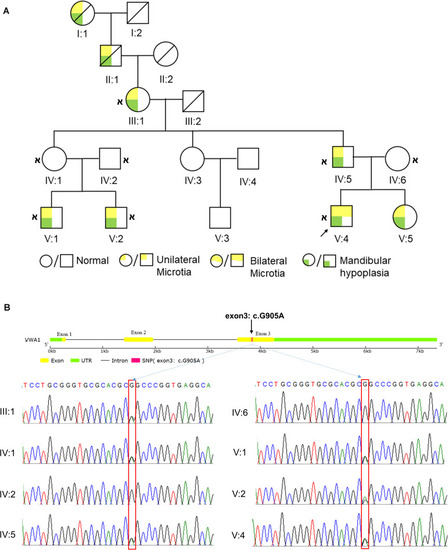
HFM pedigree and the identification of |
|
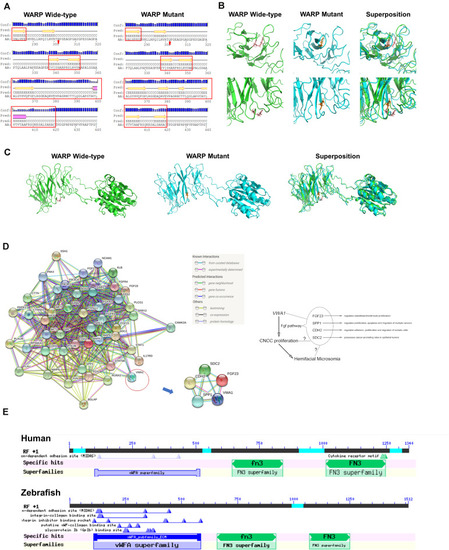
Predicted WARP structures, protein interactions and conserved domains. |
|
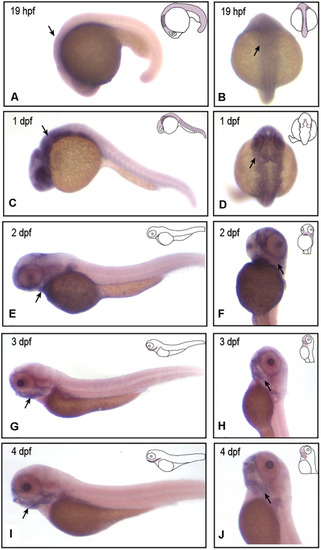
EXPRESSION / LABELING:
|
|
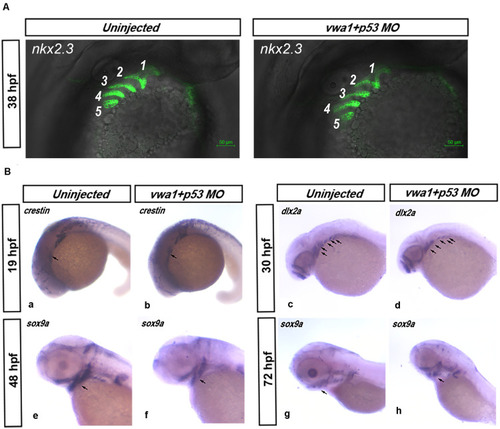
Deformities of pharyngeal cartilage in PHENOTYPE:
|
|
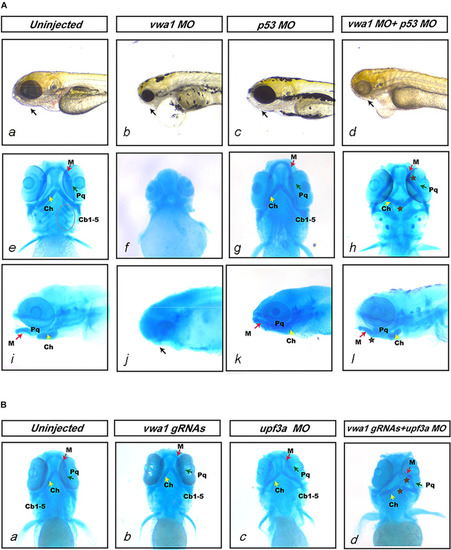
Effects of reduced EXPRESSION / LABELING:
PHENOTYPE:
|
|
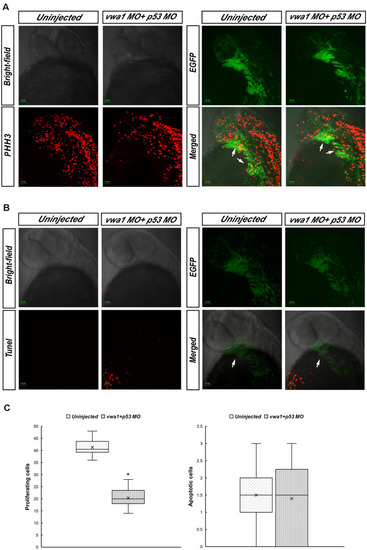
Proliferation and apoptosis of cranial neural crest cells at approximately 30 hpf. |
|
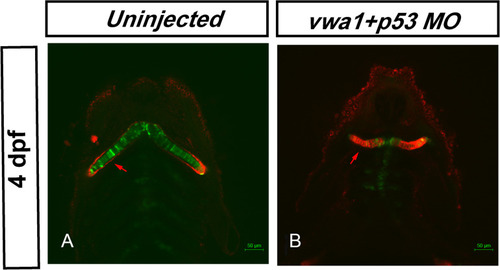
Knockdown of PHENOTYPE:
|