- Title
-
A multiorganism pipeline for antiseizure drug discovery: Identification of chlorothymol as a novel γ-aminobutyric acidergic anticonvulsant
- Authors
- Jones, A., Barker-Haliski, M., Ilie, A.S., Herd, M.B., Baxendale, S., Holdsworth, C.J., Ashton, J.P., Placzek, M., Jayasekera, B.A.P., Cowie, C.J.A., Lambert, J.J., Trevelyan, A.J., White, H.S., Marson, A.G., Cunliffe, V.T., Sills, G.J., Morgan, A.
- Source
- Full text @ Epilepsia
|
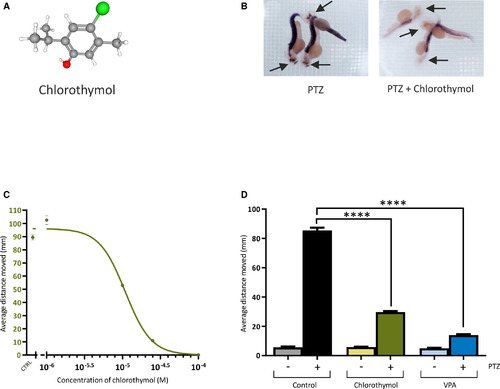
Effects of chlorothymol on pentylenetetrazol (PTZ)‐treated zebrafish. A, Chlorothymol structure (carbon = gray, hydrogen = white, oxygen = red, chlorine = green). B, In situ hybridization to detect c‐fos mRNA in 2 days postfertilization (dpf) Danio rerio larvae pretreated with dimethylsulfoxide (DMSO) vehicle only (left panel) or 25 µmol·L–1 chlorothymol in DMSO (right panel) before exposure to 20 mmol·L–1 PTZ. Arrows indicate the head of each embryo, where strong PTZ‐induced c‐fos expression is suppressed by exposure to chlorothymol. C, Chlorothymol reduces PTZ‐induced locomotor behavior in a concentration‐dependent manner. The average distance moved by 3‐dpf D rerio larvae following introduction of 20 mmol·L–1 PTZ was recorded over a 1‐hour observation period in fish pretreated with a range of chlorothymol concentrations. Data displayed show mean values ± SEM (n = 18 fish per concentration, N = 3 independent experiments). D, Chlorothymol is more potent than the prototype antiseizure drug, valproate (VPA), as it reduced the seizurelike behavior at a 100‐fold lower concentration. The average distance moved by 3‐dpf D rerio larvae following introduction of 20 mmol·L–1 PTZ was recorded over a 1‐hour observation period in fish pretreated with vehicle only (control), 25 µmol·L–1 chlorothymol, or 2.5 mmol·L–1 VPA. Data displayed show mean values ± SEM (n = 24 fish per treatment, N = 3 independent experiments). Data were analyzed using one‐way analysis of variance with Tukey multiple comparisons test (****P ≤ .0001 compared to PTZ‐treated animals) EXPRESSION / LABELING:
PHENOTYPE:
|
|
Chlorothymol has anticonvulsant activity in Caenorhabditis elegans. A, Chlorothymol reduces pentylenetetrazol (PTZ)‐induced convulsions in a concentration‐dependent manner. Seizure‐prone unc‐49(e407) mutant worms were preincubated for 15 minutes with a range of chlorothymol concentrations before incubation in the presence (green line) or absence (black dotted line) of 50 mmol·L–1 PTZ. The total number of head‐bobbing convulsions was measured over a 30‐second period and displayed as a percentage of PTZ‐treated controls ± SEM (n = 10 worms per concentration, N = 3 independent experiments). B, C elegans treated with the optimal anticonvulsant concentration of 150 µmol·L–1 chlorothymol determined in A showed a similar level of seizure reduction to the optimal concentration of valproate (VPA; 15 mmol·L–1). Data are displayed as mean ± SEM (n = 10 worms per treatment, N = 3 independent experiments) and were analyzed using one‐way analysis of variance with Tukey multiple comparison test (***P ≤ .001 compared to respective PTZ‐only treatment groups) |
|
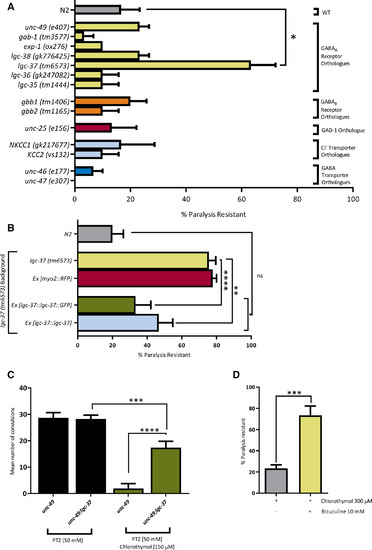
Chlorothymol acts via the Caenorhabditis elegans γ‐aminobutyric acid type A (GABAA) receptor subunit, LGC‐37. A, Genetic screen for chlorothymol‐resistant mutants. C elegans strains with mutations in genes related to GABA function were incubated for 15 minutes in 300 µmol·L–1 chlorothymol. The proportion of animals that remained paralyzed over a subsequent 30‐second interval was then scored (n = 10 worms per strain, N = 3 independent experiments). The lgc‐37(tm6573) mutant strain was found to be significantly resistant to this paralysis (*P < .05 compared to wild‐type [WT] N2 strain). B, Transgenic expression of wild‐type lgc‐37 DNA constructs reverses chlorothymol resistance in lgc‐37 mutants. Sensitivity to chlorothymol‐induced paralysis in lgc‐37 mutants expressing either green fluorescent protein (GFP)‐tagged (Ex [lgc‐37::lgc‐37::GFP]) or untagged (Ex [lgc‐37::lgc‐37]) lgc‐37 genomic clones was not significantly different from wild‐type N2 controls, whereas expression of the fluorescent marker alone (Ex [myo‐2::RFP]) had no effect (n = 15 worms per strain, N = 3 independent experiments; **P < .01, ****P ≤ .0001 compared to the lgc‐37 strain). ns, not significant; RFP, red fluorescent protein. C, Mutation of lgc‐37 reduces the anticonvulsant effect of chlorothymol. Single mutant unc‐49 and double mutant lgc‐37;unc‐49 strains were compared in the pentylenetetrazol (PTZ) assay of seizure‐related activity. Both strains produced similar levels of PTZ‐induced convulsions, but the anticonvulsant effect of chlorothymol was greatly reduced in lgc‐37;unc‐49 double mutants (n = 10 worms per treatment, N = 3 independent experiments, ***P < .001, ****P ≤ .0001 compared to the unc‐49 strain). D, The competitive GABAA antagonist, bicuculline (10 mmol·L–1) prevents paralysis induced by 300 µmol·L–1 chlorothymol (n = 10 worms per treatment, N = 3 independent experiments, ***P < .001 compared to worms treated with chlorothymol only). Data are shown as mean ± SEM and were analyzed using one‐way analysis of variance with Tukey multiple comparison test (A‐C) or by unpaired t test (D) |
|
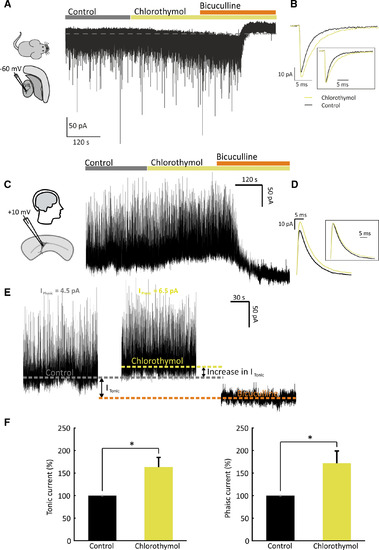
Chlorothymol increases tonic and phasic γ‐aminobutyric acidergic currents in mouse and human brain slices. A, Example whole‐cell recording from a mouse thalamic ventrobasal (VB) neuron. Inhibitory currents were isolated by holding the cell at −60 mV in voltage clamp mode in the presence of 2 mmol·L–1 kynurenic acid and 0.5 μmol·L–1 tetrodotoxin. Bath application of 54 µmol·L–1 chlorothymol induced an inward shift in the holding current, consistent with an enhancement of the resident tonic inhibitory conductance (revealed by subsequent application of 30 μmol·L–1 bicuculline). B, Superimposed miniature inhibitory postsynaptic current (mIPSC) averages obtained before and after chlorothymol application (from the same cell shown in A). The boxed inset illustrates the averaged chlorothymol current normalized to the control peak amplitude. C, Example whole‐cell recording from a human cortical neuron. Inhibitory currents were isolated by holding the cell at +10 mV in voltage clamp mode. Chlorothymol (54 µmol·L–1) and bicuculline (10 µmol·L–1) were sequentially applied to test their effects on inhibitory currents. D, Superimposed mIPSC averages obtained before and after chlorothymol application (from the cell shown in C). The boxed inset shows averaged chlorothymol current normalized to the control peak amplitude. E, Expanded timepoints from the recording in C under control, chlorothymol, and bicuculline conditions. Colored dotted lines represent the holding current under these three conditions. Bicuculline blocks both phasic (Iphasic) and tonic (Itonic) currents and hence can be used to calculate Itonic in control conditions as the difference between the holding current under control and bicuculline conditions. The increase in Itonic induced by chlorothymol was calculated as the difference in holding current between chlorothymol and control. Iphasic was calculated by averaging the traces after subtraction of the holding current (see Materials and Methods for full details). F, Population data showing that chlorothymol increased both Itonic and Iphasic in human cortical neurons. Data are shown as mean ± SEM (n = 6 brain slices) and were analyzed by paired t tests (*P < .05) |
|
A multiorganism pipeline for antiseizure drug discovery. The schematic diagram illustrates the different stages used in our approach. VB, ventrobasal |