- Title
-
Isoform-specific characterization of class I histone deacetylases and their therapeutic modulation in pulmonary hypertension
- Authors
- Chelladurai, P., Dabral, S., Basineni, S.R., Chen, C.N., Schmoranzer, M., Bender, N., Feld, C., Nötzold, R.R., Dobreva, G., Wilhelm, J., Jungblut, B., Zhao, L., Bauer, U.M., Seeger, W., Pullamsetti, S.S.
- Source
- Full text @ Sci. Rep.
|
Expression of class I HDAC isoforms are altered in different cardiopulmonary tissues from human PAH. ( |
|
Immunolocalization of class I HDAC isoforms in human lungs from donors and IPAH patients. Representative microscopic pictures of human pulmonary arteries immunostained for ( |
|
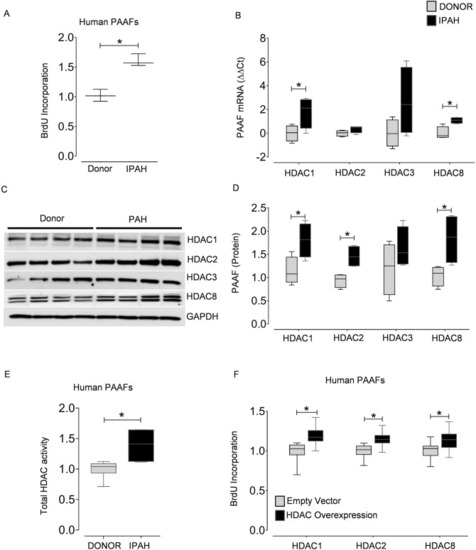
Class I HDAC isoforms are overexpressed in PAH and are associated with adventitial fibroblast proliferation. Cell proliferation was assessed by BrdU incorporation in ( |
|
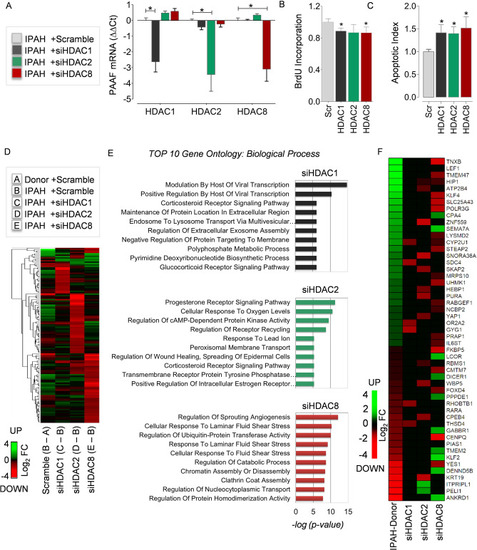
Transcriptome and pathways regulated by HDAC isoforms in PAH. RNA-interference was achieved by transient transfection of IPAH-PAAFs cultured ex vivo with validated HDAC1, HDAC2 and HDAC8 siRNAs. ( |
|
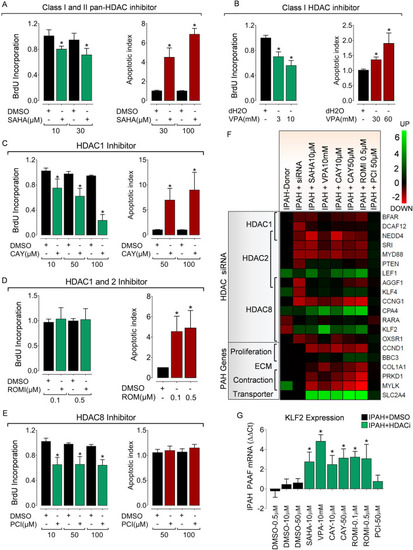
Isoform-selective HDAC activity inhibition reverses hypertensive phenotypes in PAH fibroblasts ex vivo. Pharmacological HDAC inhibition suppresses hyper-proliferative phenotype and reverses resistance to apoptosis in IPAH-PAAFs ex vivo. IPAH-PAAFs were treated with increasing concentrations of commercially available ( |
|
Regulation of class I HDACs and histone modifications by hypoxia ex vivo. ( |
|
Evaluation of isoform-selective HDAC inhibitors in chronic hypoxia-induced PH and RV hypertrophy in vivo. ( |