- Title
-
Mitochondria in Embryogenesis: An Organellogenesis Perspective
- Authors
- Arribat, Y., Grepper, D., Lagarrigue, S., Richard, J., Gachet, M., Gut, P., Amati, F.
- Source
- Full text @ Front Cell Dev Biol
|
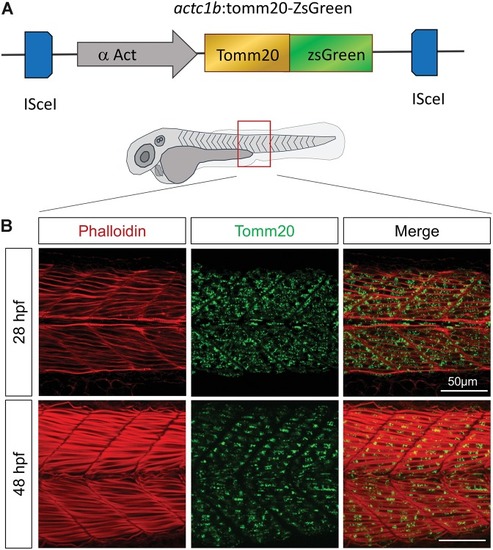
Tomm20-zsGreen transgenic zebrafish represent a relevant model to decipher mitochondrial modifications during embryogenesis. |
|
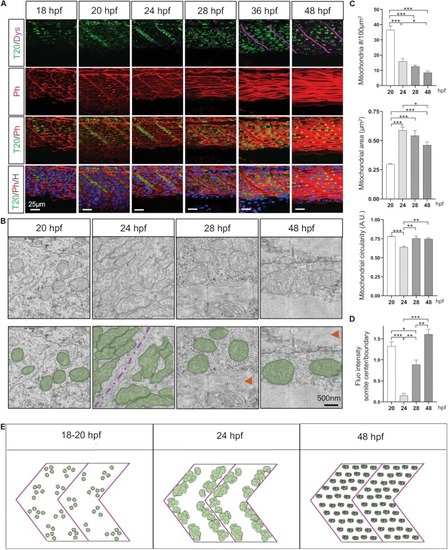
Mitochondria network adaptation follows three patterns of change through embryogenesis. EXPRESSION / LABELING:
|
|
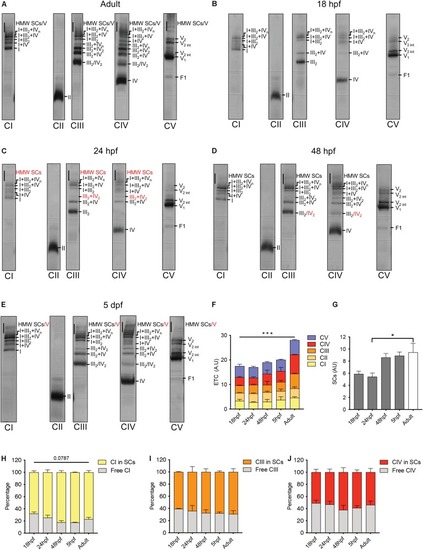
Electron transport chain supercomplexes are qualitatively stable throughout zebrafish development. EXPRESSION / LABELING:
|
|
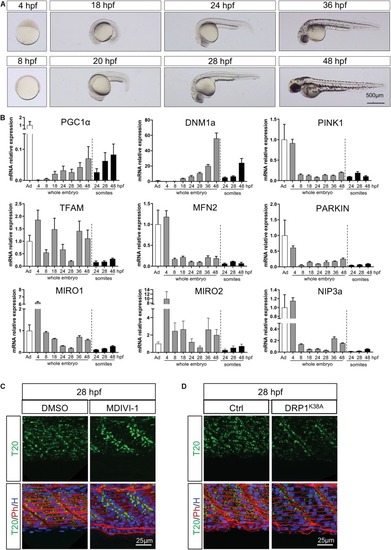
Temporal configuration of mitochondrial biogenesis, fusion, fission, mitophagy and transport through embryogenesis. |
|
Neuronal stimulation and muscle contractions do not mediate mitochondrial patterning. |
|
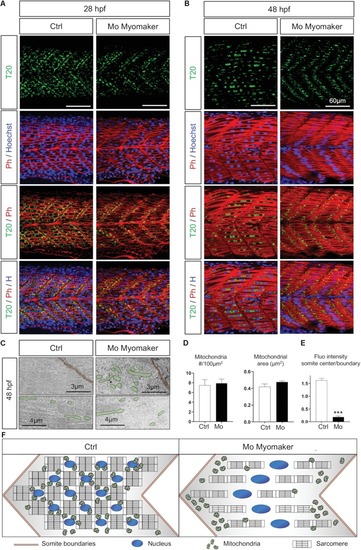
Mitochondrial network maturation is conditional to myoblast fusion. |
|
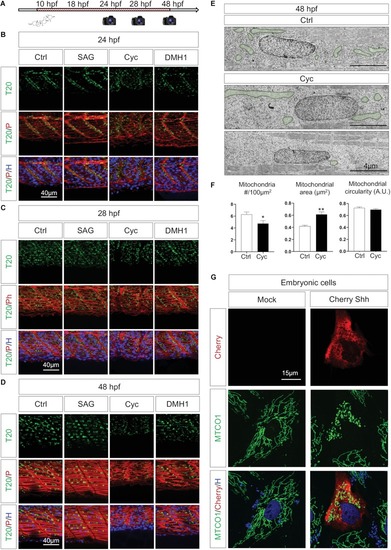
Shh signaling synchronizes tissue formation and mitochondria network maturation. |
|
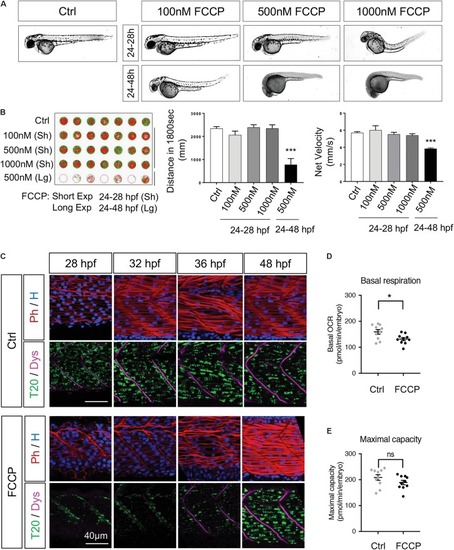
Mitochondrial quality is indispensable for embryogenesis. |