- Title
-
Encapsulation of a TRPM8 Agonist, WS12, in Lipid Nanocapsules Potentiates PC3 Prostate Cancer Cell Migration Inhibition through Channel Activation
- Authors
- Grolez, G.P., Hammadi, M., Barras, A., Gordienko, D., Slomianny, C., Völkel, P., Angrand, P.O., Pinault, M., Guimaraes, C., Potier-Cartereau, M., Prevarskaya, N., Boukherroub, R., Gkika, D.
- Source
- Full text @ Sci. Rep.
|
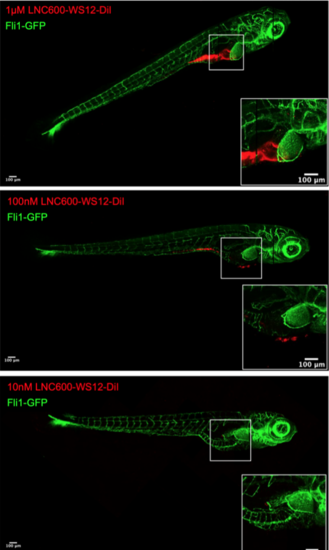
Confocal analysis of LNC600-WS12 uptake in zebrafish. LNC600-WS12 is labeled with DiI (red), and the results are shown for experiments performed in which 1 µM (A), 100 nM (B) or 10 nM (C) was used to treat zebrafish embryos for 6 days before the embryos were fixed. Confocal analysis showing the internalization of DiI-labeled LNC600-WS12 in the zebrafish. Zooms show the presence of LNC600-WS12 into the yolk sacs of zebrafish after treatment with 1µM and 100nM of LNC600-WS12. |
|
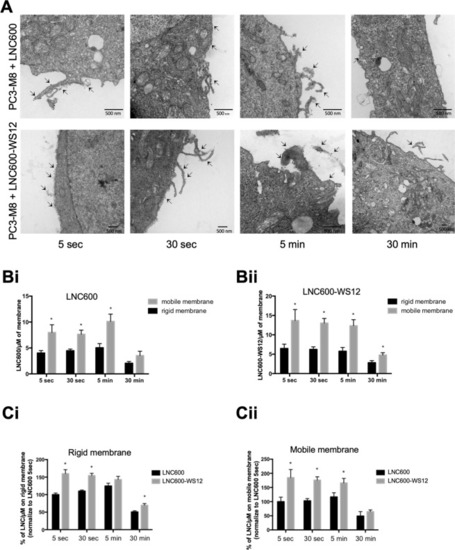
Plasma membrane penetration and dynamic localization of lipid nanocapsules. ( |
|
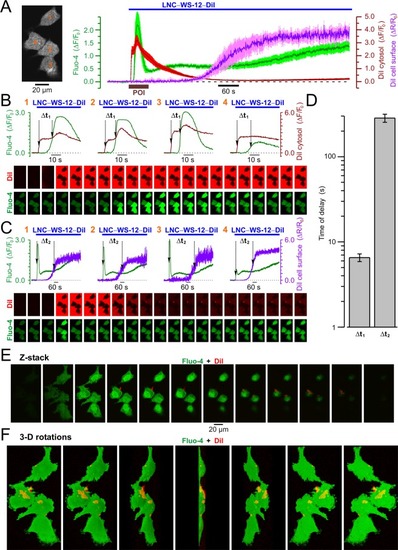
Confocal visualization of DiI/WS12-containing lipid nanocapsules (LNC600-WS12-DiI) and concurrent LNC-induced [Ca2±]i responses in PC3 cell expressing stably TRPM8. ( |
|
|