- Title
-
Glucocorticoids Target Ependymal Glia and Inhibit Repair of the Injured Spinal Cord
- Authors
- Nelson, C.M., Lennon, V.A., Lee, H., Krug, R.G., Kamalova, A., Madigan, N.N., Clark, K.J., Windebank, A.J., Henley, J.R.
- Source
- Full text @ Front Cell Dev Biol
|
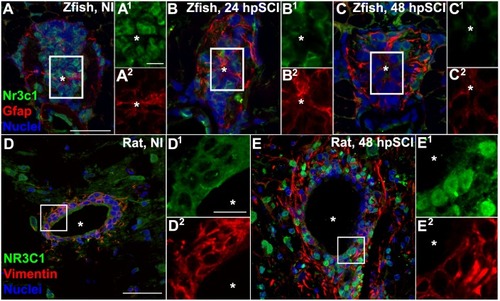
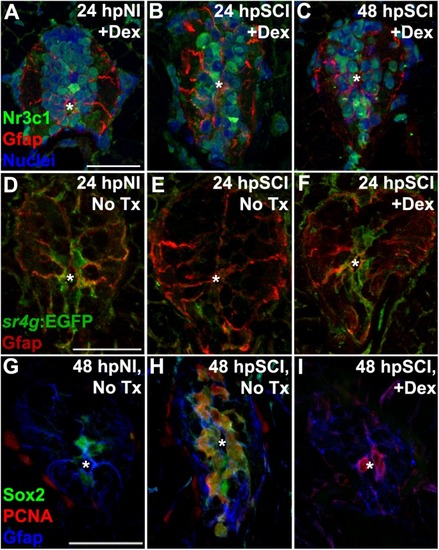
Spinal cord injury stimulates differential Nr3c1 expression by ependymal glia in zebrafish and rats. |
|
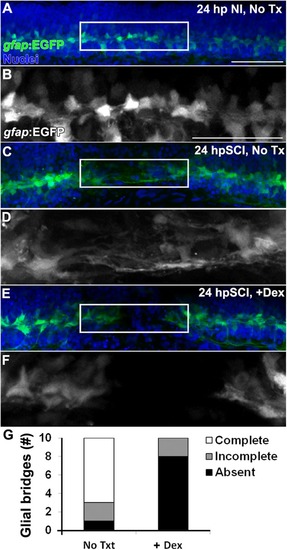
Glucocorticoids inhibit the formation of |
|
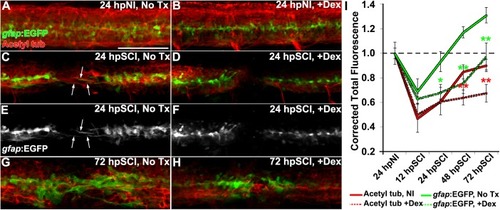
Glucocorticoids suppress glial bridges and axon regrowth. |
|
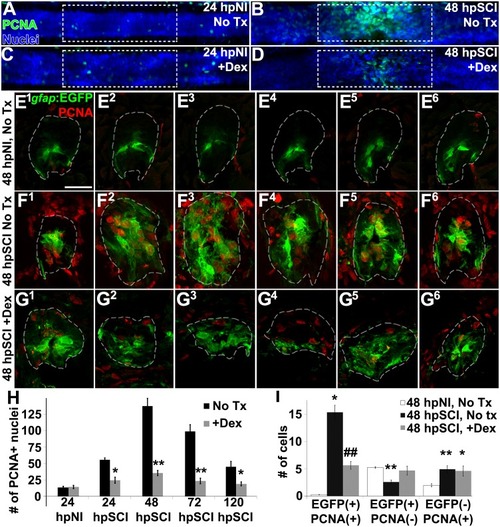
Glucocorticoids inhibit ependymal glial proliferation following SCI. |
|
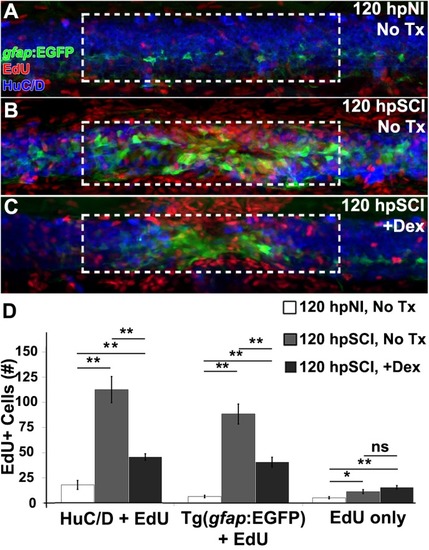
Glucocorticoids suppress neurogenesis. |
|
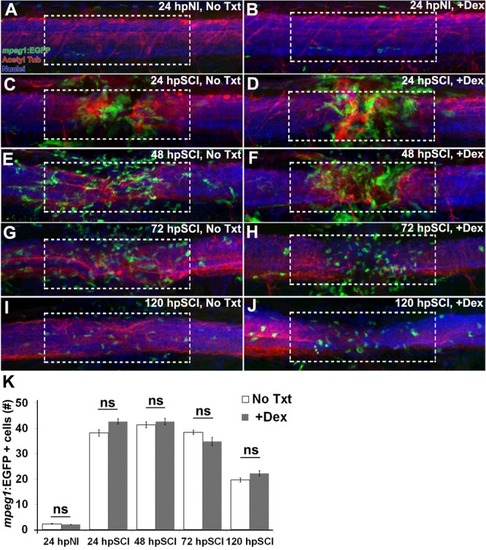
Responses of hematogenous cells and microglia to SCI and Dex treatments. |
|
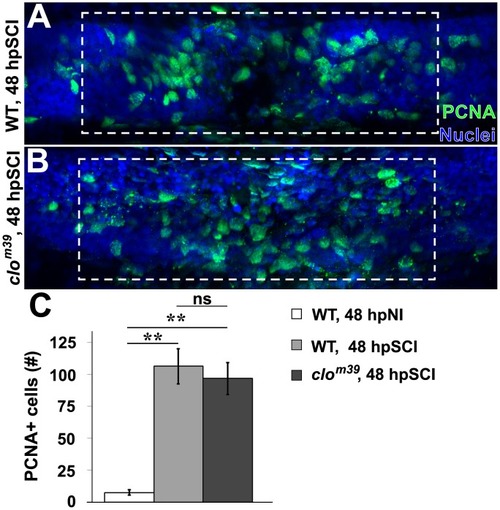
SCI-induced proliferation occurs without hematogenic or microglial responses. |
|
Direct effects of glucocorticoids in ependymal glia. |