- Title
-
CPSF1 mutations are associated with early-onset high myopia and involved in retinal ganglion cell axon projection
- Authors
- Ouyang, J., Sun, W., Xiao, X., Li, S., Jia, X., Zhou, L., Wang, P., Zhang, Q.
- Source
- Full text @ Hum. Mol. Genet.
|
Knockdown of PHENOTYPE:
|
|
Co-injection of PHENOTYPE:
|
|
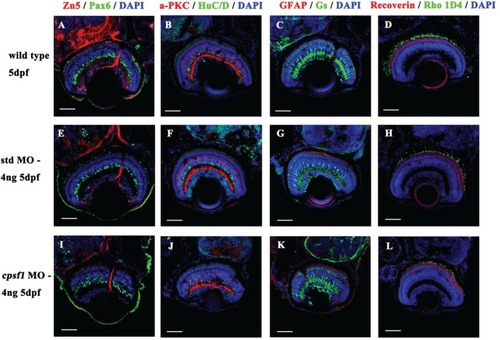
Retinal cells developed normally in WT larvae EXPRESSION / LABELING:
|
|
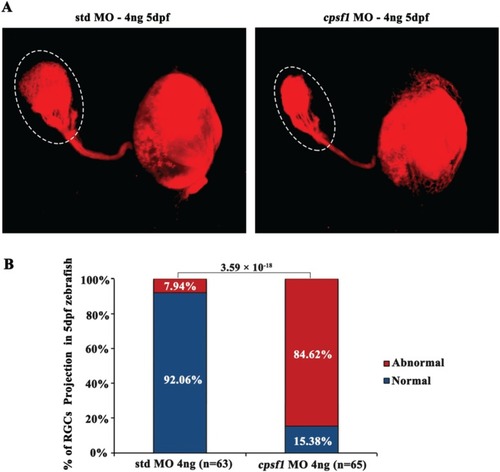
RGC axon projection to the tectum was abnormal in PHENOTYPE:
|
|
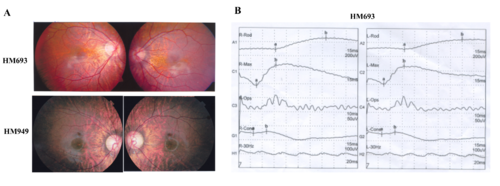
. Available fundus changes of the probands HM693 and HM949 and ERG recording of the proband HM693. (A) Typical fundus changes of high myopia, including an optic nerve head crescent and a “tigroid” appearance of the posterior retina. (B) ERG recording of the proband HM693 showed severely reduced cone responses and mildly reduced rod responses |
|
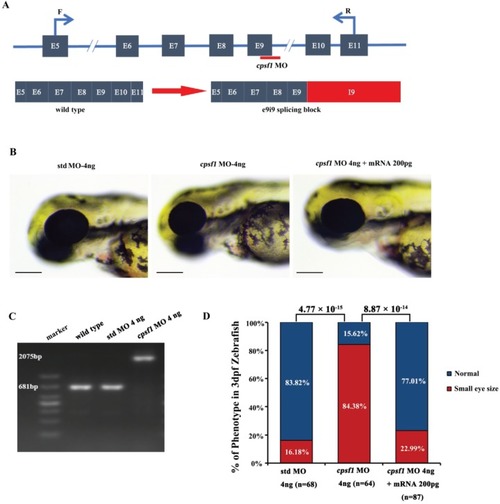
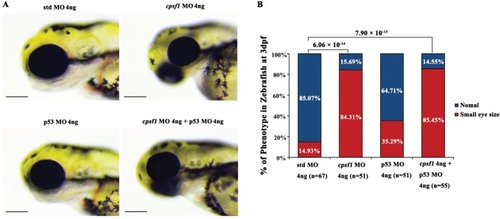
Knockdown of cpsf1 at different doses in zebrafish caused differences in ocular morphogenesis. (A) Phenotype of MO-injected zebrafish. Upper: microinjection with 2 ng, 4 ng, or 6 ng of std MO; lower: microinjection with 2 ng, 4 ng, or 6 ng of cpsf1 MO. The data showed that body and eye size were normal after injecting 2 ng, 4 ng, or 6 ng of std MO. Three phenotypes were observed in the cpsf1 morphants and classified based on degree (mild, moderate or severe) following injection with 2 ng, 4 ng or 6 ng of cpsf1 MO. (B) Quantification of small eye size proportions in zebrafish at 3 dpf. The eye diameter (eye size) was 290.58 ± 17.94 μm (n=45) for zebrafish injected with std MO (2 ng) and 289.58 ± 28.84 μm (n= 60) for zebrafish injected with cpsf1 MO (2 ng); 285.28 ± 12.25μm (n=51) for zebrafish injected with std MO (4 ng) and 250.03 ± 32.31 μm (n=74) for zebrafish injected with cpsf1-MO (4 ng); and 285.06 ± 15.80 μm (n=57) for zebrafish injected with std MO (6 ng) and 212.64 ± 28.68 μm (n=83) for zebrafish injected with cpsf1-MO (6 ng). The data showed that the proportion of small eye size was significantly increased in cpsf1 morphants injected with 4 ng and 6 ng of cpsf1 MO than in those injected with the std control (P= 4.66 × 10-10 for 4 ng and P= 9.17 × 10-17 for 6 ng), but there was no difference between the cpsf1 morphants injected with 2 ng of cpsf1 MO and those injected with the std control (P = 0.88) based on Chi-square test (P<0.05 was considered as statistically significant). The number of zebrafish injected in each group is indicated under each column. |
|
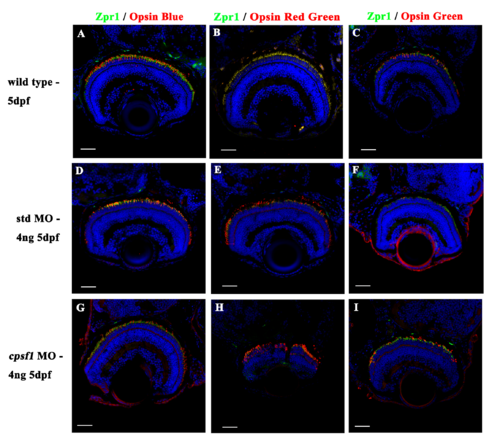
Photoreceptor cells developed normally in wild-type larvae (A-C), std MO larvae (D-F), and cpsf1 morphants (G-I). (A, D, G): Co-labelling with anti-Zpr1 (for double-cone photoreceptor cells, green) and anti-Opsin Blue (for blue cone photoreceptor cells, red) antibodies showed that the blue cone photoreceptor cells were mature. (B, E, H): Co-labelling with anti-Zpr1 (for double-cone photoreceptor cells, green) and anti-Opsin Red/Green (for red/green cone photoreceptor cells, red) antibodies indicated that the red and green cone photoreceptor cells developed normally. (C, F, I): Co-labelling with anti-Zpr1 (for double-cone photoreceptor cells, green) and anti-Opsin Green (for green cone photoreceptor cells, red) antibodies demonstrated that the green cone photoreceptor cells attained maturity. EXPRESSION / LABELING:
|