- Title
-
Collagenolytic Activity Is Associated with Scar Resolution in Zebrafish Hearts after Cryoinjury
- Authors
- Gamba, L., Amin-Javaheri, A., Kim, J., Warburton, D., Lien, C.L.
- Source
- Full text @ J Cardiovasc Dev Dis
|
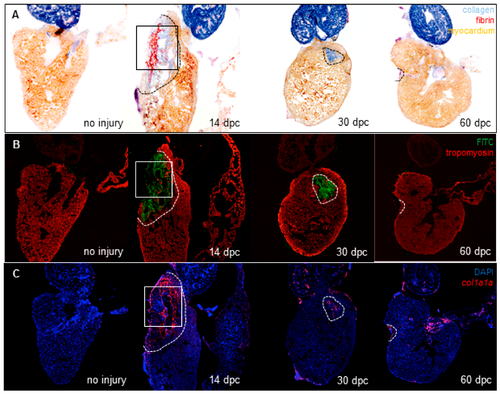
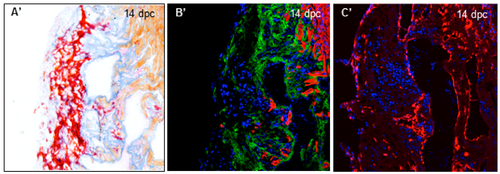
Collagenolytic activity and fibroblast activation during heart regeneration. (A) Wound area visualized with AFOG staining of frozen sections from uninjured hearts (n = 3) or hearts at 14 dpc (n = 7), 30 dpc (n = 8) and 60 dpc (n = 6) (fibrin in red, collagen in blue, and counterstaining in orange); (B) Confocal imaging of consecutive sections from hearts stained in (A). Green fluorescence represents the release of fluorescein activity due to the degradation of DQ-collagen substrate. Tropomyosin immunostaining is used as counterstaining (red fluorescence); (C) col1a1a expression by fluorescent in situ hybridization on consecutive sections from the same hearts shown in (A,B). col1a1a mRNA in red, nuclei in blue (DAPI). Comparison of collagenolytic activity (B’) and fibroblasts (C’) with collagen (A’) on consecutive sections of the 14 dpc heart at a 20× magnification (magnification of the boxes in (A–C) respectively). Dashed lines represent the wound area. |
|
Collagenolytic activity and fibroblast activation during heart regeneration. (A) Wound area visualized with AFOG staining of frozen sections from uninjured hearts (n = 3) or hearts at 14 dpc (n = 7), 30 dpc (n = 8) and 60 dpc (n = 6) (fibrin in red, collagen in blue, and counterstaining in orange); (B) Confocal imaging of consecutive sections from hearts stained in (A). Green fluorescence represents the release of fluorescein activity due to the degradation of DQ-collagen substrate. Tropomyosin immunostaining is used as counterstaining (red fluorescence); (C) col1a1a expression by fluorescent in situ hybridization on consecutive sections from the same hearts shown in (A,B). col1a1a mRNA in red, nuclei in blue (DAPI). Comparison of collagenolytic activity (B’) and fibroblasts (C’) with collagen (A’) on consecutive sections of the 14 dpc heart at a 20× magnification (magnification of the boxes in (A–C) respectively). Dashed lines represent the wound area. |
|
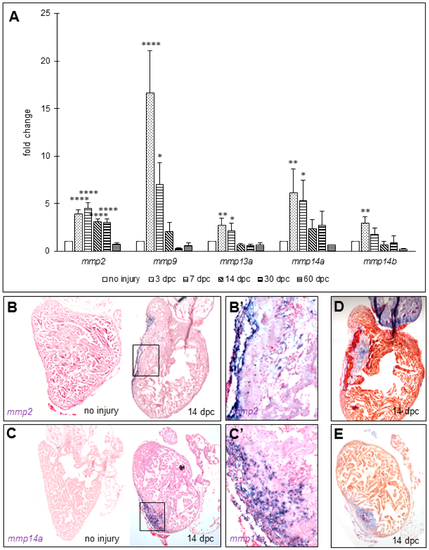
MMPs transcripts increase in zebrafish ventricles after cryoinjury. (A) RT-quantitative PCR for mmp2, mmp9, mmp13a, mmp14a, and mmp14b in ventricles at several time points after cryoinjury (six to eight ventricles per time point). Significant difference with uninjured hearts: **** p < 0.0001, ** p < 0.01, * p < 0.05; (B,C) in situ hybridization in uninjured and 14 dpc hearts of mmp2 (n = 1 and 2, respectively) and mmp14a (n = 2 and 2, respectively) transcripts respectively; (B’,C’) Magnification of the boxes in (B,C), respectively; (D,E) AFOG staining on consecutive sections of 14 dpc hearts shown in (B,C) respectively for comparison with the wound area (fibrin in red, collagen in blue, and counterstaining in orange). |
|
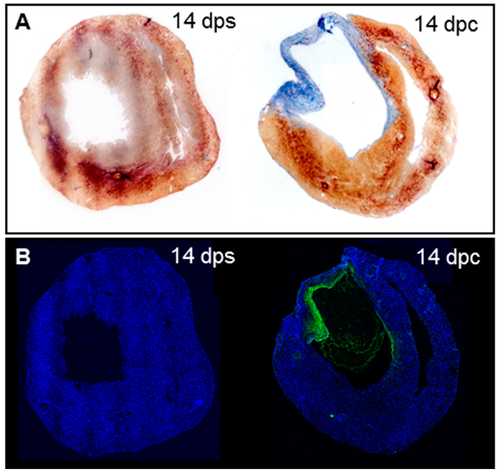
Collagenolytic activity in the scar of neonatal mouse hearts after cryoinjury. (A) AFOG staining on neonatal mouse hearts at 14 dps (n = 3) and 14 dpc (n = 4); (B) In situ zymography on consecutive sections from hearts in (A). |

Unillustrated author statements EXPRESSION / LABELING:
|