- Title
-
Prolyl Isomerase Pin1 Regulates Axon Guidance by Stabilizing CRMP2A Selectively in Distal Axons.
- Authors
- Balastik, M., Zhou, X. Z., Alberich-Jorda, M., Weissova, R., Žiak, J., Pazyra-Murphy, M. F., Cosker, K. E., Machonova, O., Kozmikova, I., Chen, C. H., Pastorino, L., Asara, J. M., Cole, A., Sutherland, C., Segal, R. A., Lu, K.P.
- Source
- Full text @ Cell Rep.
|
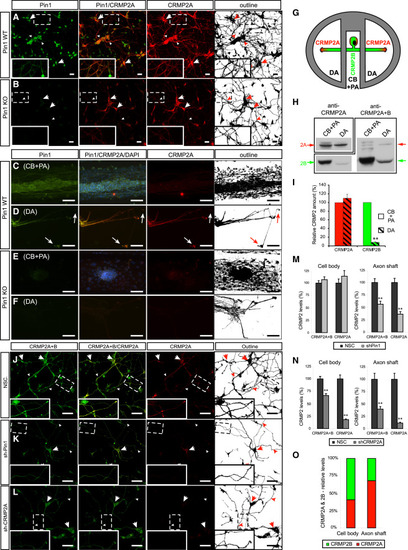
Pin1 Stabilizes CRMP2A Selectively in the Distal Neurites of Primary Neurons (A and B) Pin1 KO reduces CRMP2A selectively in the distal neurites of primary neurons. CRMP2A (red) and Pin1 (green) immunostaining in Pin1 WT and KO primary cortical neurons at 3 DIV. (A) CRMP2A is expressed strongly in the soma (arrows) as well in the neurites (arrowheads) and co-localizes with Pin1 in the neurites (insets). In the Pin1 KO neurons (B), CRMP2A levels are lower in the neurites (arrowheads), but its levels in the somas are comparable to WT (arrows). (C-F) Pin1 KO reduces CRMP2A in axons. Using rat Pin1 WT DRG compartmental cultures, Pin1 was detected both in the cell body and proximal axon (CB + PA) compartment (C) and in distal axons (DA) (D) by double immunostaining, while higher expression of CRMP2A (co-localizing with Pin1) was detected in DA close to growth cones (D, arrows) when compared to the CB + PA (C). In the Pin1 KO DRG neurons, CRMP2A expression in the DA (F), but not in the CB + PA region (E), is significantly reduced. (G-I) The relative level of CRMP2A increases in distal axon region. Lysates collected from DA and CB + PA compartments of rat primary DRG compartment cultures (G) were analyzed by western blotting using CRMP2A and total CRMP2A+B antibodies (H). Semiquantitative analysis of CRMP2A and CRMP2B levels shows significant reduction of CRMP2B levels in distal axons but no significant reduction of CRMP2A levels (I). (J-L) Pin1 KD reduces CRMP2A and total CRMP2 selectively in neurites. Pin1 WT primary cortical neurons were infected with non-silencing (NSC), Sh-Pin1, or Sh-CRMP2A lentiviruses and immunostained for CRMP2A (red) or total CRMP2 (CRMP2A+B) (green). In NSC neurons, high levels of CRMP2A and total CRMP2 (CRMP2A+B) were detected in both the neurites (arrowhead) and the soma (arrow) (J). Pin1 KD significantly reduced CRMP2A and total CRMP2 levels in neurites (arrowhead), but not in cell bodies (arrow) (K). CRMP2A KD significantly decreases CRMP2A and total CRMP2 levels in neurites (arrowhead) as well as cell bodies (arrows) (L). (M and N) Quantification of total CRMP2 and CRMP2A levels in Pin1 KD (shPin1) and non-silencing shRNA (NSC) control neurons in the neuronal cell body and in the axon shafts (M). (O) Quantification of total CRMP2B and CRMP2A levels in CRMP2A KD (shCRMP2A) and control (NSC) neurons in the neuronal cell body and axon shafts. Relative distribution of CRMP2A versus CRMP2B in the cell body and distal axons calculated from (N). Scale bars represent 20 µm (A and B) and 50 µm (C-F and J-L). Data are means ± SEM; p < 0.0001. See also Figure S2. PHENOTYPE:
|
|
KD/KO of Pin1 Reduces Axon Growth in Primary Neurons and Is Fully Rescued by CRMP2A Overexpression Primary cortical neuron cultures were derived from embryos of three independent Pin1 WT (A-F) and KO (G-L) mouse littermates and infected with lentiviruses expressing control vector (A and G), FLAG-Pin1 (B and H), Sh-CRMP2A (C and I), Sh-Pin1 (D and J), or non-silencing shRNA (NSC) (E and K) lentiviruses. Their axon length was determined at 3 DIV by immunostaining for the axon marker tau (green) and the dendrite marker MAP2 (yellow). Axon tracings are shown in violet. (F) and (L) show the means ± SEM values calculated from quantification of at least two optical fields and at least 50 neurons in each experiment. In Pin1 WT neurons, while overexpression of Pin1 had no significant effect, KD of CRMP2A or Pin1 in Pin1 WT neurons significantly reduced axon length (**p < 0.0001). In Pin1 KO neurons, overexpression of Pin1 completely rescued axon length, but KD of CRMP2A or Pin1 did not significantly reduce axon length, although Sh-CRMP2A neurons had slightly shorter axon. (M-P) CRMP2A overexpression fully rescues shortened axon length in Pin1 KO neurons. Pin1 WT (M and O) and KO (N and P) primary cortical neurons were co-transfected with GFP and vector control (M and N) or GFP and FLAG-CRMP2A (O and P) and axon growth was analyzed at 7 DIV in GFP-positive neurons. Outlines of the neurons are shown in lower right boxes. Upper right panel indicates quantification of the axon lengths as mean ± SEM (*p < 0.05, **p < 0.001). Scale bars, 100 µm. (M, O, and P) are composite images of four, six, and seven images, respectively. See also Figure S3. |
|
Pin1 KO Increases Sensitivity to Sema3A-Induced Growth Cone Collapse in Primary Dorsal Root Ganglia Neurons (A and B) Sema3A induces colocalization of high levels of Pin1 and CRMP2A in the vicinity of growth cones (arrows) in Pin1 WT, but not Pin1 KO, DRG axons. Pin1 WT (A) and KO (B) primary DRG neurons were treated with 0.1 nM Sema3A for 30 min, followed by double immunostaining for Pin1 (green) and CRMP2A (red). (C-J) Pin1 KO increases sensitivity to Sema3A-induced growth cone collapse. Pin1 WT (C-E) and KO (F-H) primary DRG neurons were treated with different concentrations of Sema3A for 30 min, fixed, and triple immunostained with anti Pin1, β-actin, and β-tubulin antibodies, followed by growth cone collapse analysis, with the percentage of growth cone collapse being shown in (J). Red dots, collapsed growth cones; green dots, intact growth cones. Intensity of Pin1 immunostaining in DRG growth cones (C) significantly increases upon low (non-collapsing) Sema3A stimulation (D) and is reduced upon high Sema3A stimulation (E); the quantification after normalization to ²-tubulin levels is shown (I). (K and L) Stimulation with LPA induces similar growth cone collapse in Pin1 WT and KO DRG neurons (L) and does not affect Pin1 levels in the growth cones (K). (M-Q) Pin1 KO significantly increases sensitivity to Sema3A-induced growth cone collapse in collagen 3D co-cultures. SH-SY5Y cells were transfected with empty vector (M and O) or Sema3A expression vector (N and P) and co-cultured with Pin1 WT (M and N) or KO (O and P) DRG. Proximal/distal axon length ratio was measured (N and P, arrows) upon NF-M immunostaining and quantified (Q). Scale bars represent 50 µm (A and B), 20 µm (C-H), and 500 µm (M-P); *p < 0.05; **p < 0.0001. Values are means ± SEM. See also Figure S4. |
|
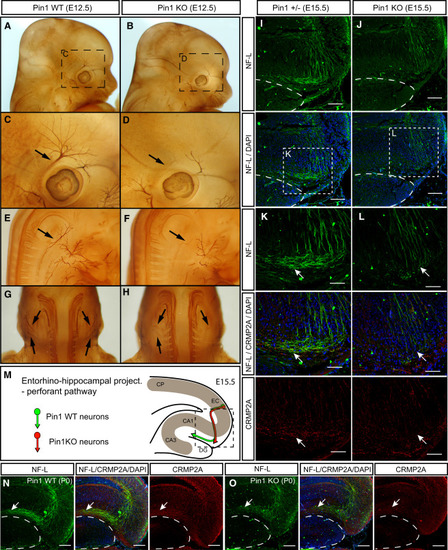
Pin1 KO Leads to Developmental Axon Growth Defects in the Peripheral Nervous System and CNS (A-H) Axons of the cranial and spinal nerves are less extensive and complex in Pin1 KO embryos. The cranial (A-D) and spinal (E-F) nerves were analyzed in E12.5 Pin1 WT (A, C, E, and G) and KO (B, D, F, and H) embryos by whole-mount immunohistochemistry for neurofilaments. In Pin1 KO embryos, stunted neurite processes are found in the ophthalmic branch of the trigeminal nerve (B and D, arrows) and in the lateral branches of the cervical spinal nerves (F and H, arrows). (I-M) Entorhino-hippocampal perforant projections are significantly shorter in Pin1 KO embryos at E15.5. Horizontal sections of E15.5 embryos were stained for neurofilament light subunit (NF-L) (green) to trace growth of entorhinal perforant projections toward the stratum lacunosum-moleculare (s.l-m) of the hippocampus proper in Pin1+/- (I) and KO (J) mice. The dashed lines indicate borders of the developing dentate gyrus. (K and L) Pin1 KO shorter perforant projections are associated with reduced CRMP2A level (red) in growth cones, as indicated by arrows. (M) Schematic drawing of the entorhino-hippocampal perforant pathway at E15.5 in the presence or absence of Pin1 as shown in (I)-(L). CP, cortical plate; EC, entorhinal cortex; DG, dentate gyrus; CA1, CA3, hippocampal regions. (N and O) Developmental defects are corrected later during development of Pin1 KO mice. Entorhinal perforant projections are detected in s.l-m of both Pin1 WT (N, arrow) and KO (O, arrow) newborn mice. Lower levels of CRMP2A are present in perforant projections in s.l-m in Pin1 KO mice (O). Scale bars represent 100 µm (I, J, N, and O) and 50 ¼m (K and L). See also Figure S5. |
|
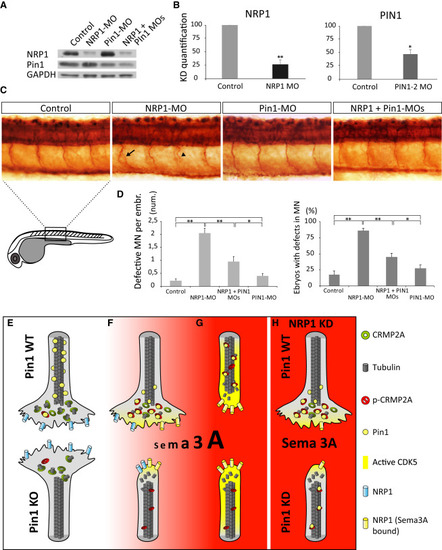
Pin1 Regulates Sema3A Signaling In Vivo (A and B) Efficiency of single- and double-morpholino KD of zPin1 and NRP1 in 24-hr zebrafish embryos analyzed by immunoblotting (A), with quantification in (B). (C) Whole-mount immunostaining of acetylated tubulin in 1-day-old zebrafish embryos demonstrates increased incidence of developmental motor neurons defects, e.g., aberrant branching (arrow) or truncated growth (arrowhead), upon NRP1 KD, which is reduced upon co-injection of Pin1-MO. (D) Quantification of developmental defects in morpholino-injected zebrafish embryos. Control embryos and Pin1-MO injected do not significantly differ in percentage of defective embryos (17% versus 27%, respectively) or average number of defects per embryo (0.22% versus 0.4%, respectively). NRP1 KD induces defective development in 85% of embryos, with an average of 2.05 defects per embryo. Co-injection of Pin1-MO significantly reduces number of defective embryos (44%) as well as the average number of defects per embryo (0.95). (E-H) A model of Pin1’s role in Sema3A-driven axonal growth and retraction. Values are means ± SEM. |
|
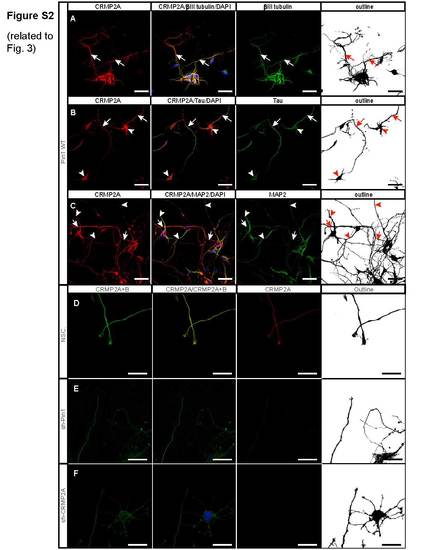
Knockdown of Pin1 or CRMP2A leads to reduction of total CRMP2 level in the vicinity of growth cones. Related to Figure 3 (A) CRMP2A is strongly expressed in primary cortical neurons, as detected by neuron marker βIII tubulin (arrows). (B) Strongest CRMP2A staining is present in distal axons (arrows), as identified by co-immunostaining with tau. CRMP2A is also present in neuronal cell bodies (arrowheads). (C) CRMP2A is present also in dendrites of primary cortical neurons (arrowheads), as identified by the dendritic marker MAP2, even though its level seems lower when compared to CRMP2A level in distal axons (arrows). Knockdown of Pin1 (E) or CRMP2A (F) in the Pin1 WT primary cortical neurons significantly reduces level of CRMP2A as well as total CRMP2 (CRMP2A+B) in the vicinity of growth cones, when compared to control cortical neurons infected with a non-silencing lentiviral vector (D). (Scale bars A – C 50 µm, D – F 20 µm). |
|
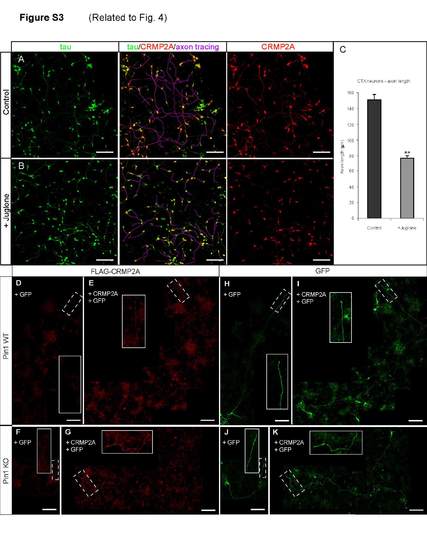
Inhibition of Pin1 isomerase activity reduces axon growth and CRMP2A axon levels in primary cortical neurons. Overexpressed FLAG-tagged CRMP2A accumulates in distal axons. Related to Figure 4 Treatment of 1 DIV cortical neurons with Juglone, an inhibitor of Pin1 isomerase activity for 3 days (B) reduces CRMP2A levels in axons and axon growth as analyzed by CRMP2A and tau double immunostaining. (A) Untreated control. (C) Quantification of axon length. (p < 2e-06, means ± SEM values shown). (E-K) Expression of FLAG-tagged CRMP2A leads to CRMP2A accumulation in distal axons both in Pin1 WT (E) and KO (G) primary cortical neurons, as visualized by anti-FLAG antibodies. No FLAG signal was detected in vectortransfected Pin1 WT (D) or KO (F) neurons. (H-K) GFP signal used to trace the transfected neurons in (D-G). (Scale bars 100 µm), (D and H, E and I, G and K are composite images of 4, 7 and 6 images, respectively). |
|
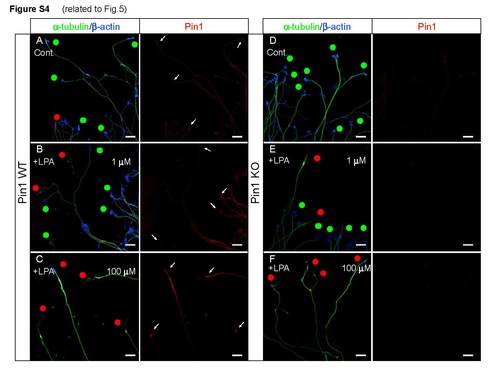
Pin1 KO does not increase sensitivity to LPA treatment. Related to Figure 5 Primary Pin1 WT and KO cortical neurons were treated with 0.1, 1, 10 and 100 µM LPA, fixed and triple immunostained with anti-Pin1, β-tubulin and β-actin antibodies, followed by growth cone collapse analysis, revealing a similar percentage of collapsed growth cones in Pin1 WT (A-C) and KO (D-F) DRGs. Red dots - collapsed growth cones; green dots - intact growth cones. Similarly, intensity of Pin1 immunostaining in Pin1 WT DRG growth cones (A-C) is not significantly different compared to Pin1 KO DRGs upon low (B, E) or high (C, F) LPA stimulation and normalization to β-tubulin levels. (Scale bars: 20 µm). |
|
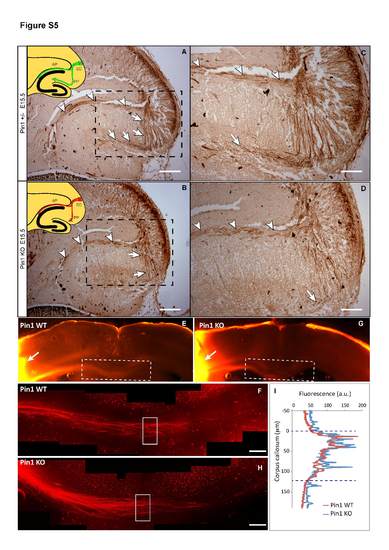
Alvear pathway of the enthorino-hippocampal projections at E15.5 Pin1 KO embryos and fasciculation of somatosensory (S1) axons at midline of corpus callosum in adult Pin1 KO mice. Related to Figure 6 NF-L immunostaining of E15.5 brain horizontal sections shows normal development of the Pin1 KO (B, D) alvear projections (arrowheads), while growth of perforant fibers is significantly reduced (arrows) when compared to Pin1 WT littermates (A, C). The fasciculation of S1 axons in midline corpus callosum is similar in Pin1 WT (E, F) and Pin1 KO (G, H) adult mice (3 month old) (arrows indicates the place of DiI crystal insertion). (I) Fluorescence intensity of S1 axons along the D-V axis at the midline shows similar distribution and diameter of the axon bundle in Pin1 WT and Pin1 KO mice. EC-entorhinal cortex, PP-perforant pathway, AP-alvear pathway (Scale bars: A, B 200 µm; C, D, F, H 100 µm). (F, H are composite images of 6 and 7 images, respectively). |