- Title
-
Effects of copper oxide nanoparticles on developing zebrafish embryos and larvae
- Authors
- Sun, Y., Zhang, G., He, Z., Wang, Y., Cui, J., Li, Y.
- Source
- Full text @ Int. J. Nanomedicine
|
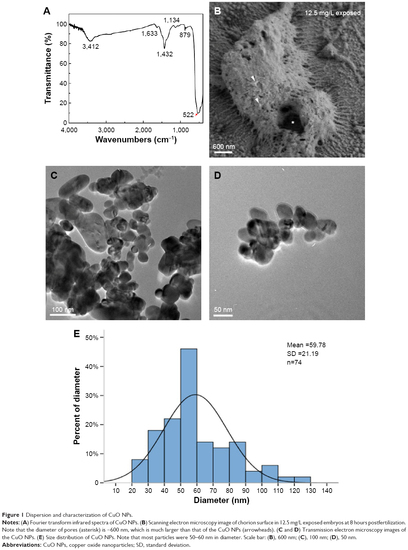
Dispersion and characterization of CuO NPs. |
|
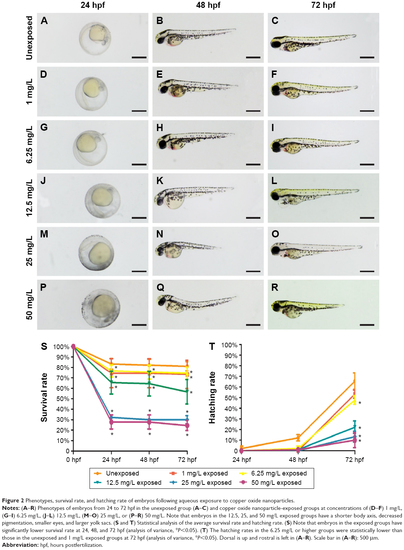
Phenotypes, survival rate, and hatching rate of embryos following aqueous exposure to copper oxide nanoparticles. |
|
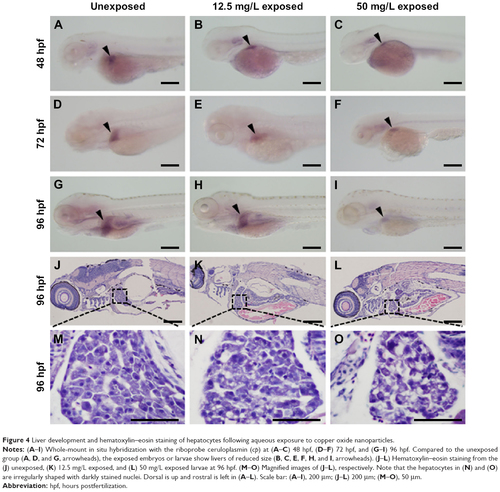
Liver development and hematoxylin–eosin staining of hepatocytes following aqueous exposure to copper oxide nanoparticles. |
|
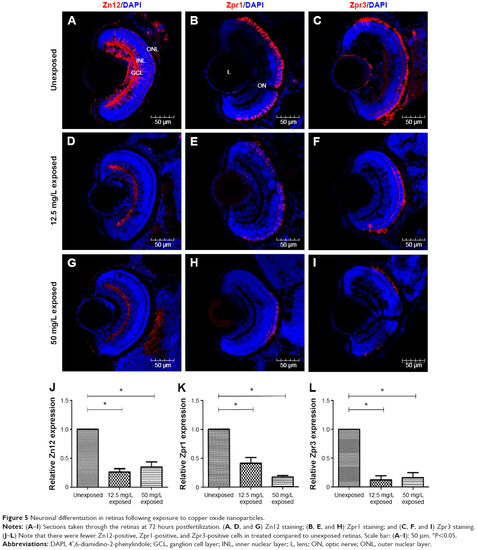
Neuronal differentiation in retinas following exposure to copper oxide nanoparticles. |
|
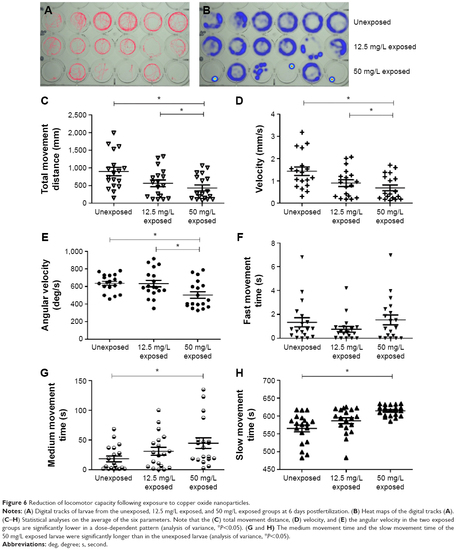
Reduction of locomotor capacity following exposure to copper oxide nanoparticles. |