- Title
-
miR-430 regulates oriented cell division during neural tube development in zebrafish
- Authors
- Takacs, C.M., Giraldez, A.J.
- Source
- Full text @ Dev. Biol.
|
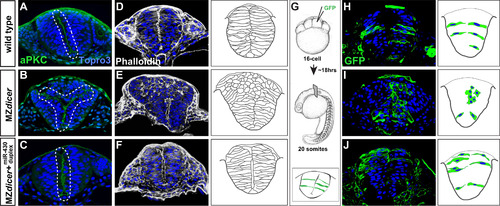
miR-430 is required for neural tube morphogenesis. Transverse sections through the hindbrain region of 20-somite stage embryos. Topro3, nuclear stain. Wildtype neural progenitors (A, D and H) display apical localization of aPKC and bilateral organization along the presumptive neural midline (A), display normal subcellular localization of F-actin (D), and acquire elongated cell morphology (H). In contrast, MZdicer neural progenitors (B, E and I) form ectopic apical membranes (B), display abnormal F-action organization (E), and fail to establish contact with lateral membrane and acquire epithelial character in dorsal regions (I). (C, F and J) Neural progenitors from MZdicer embryos injected at the 1-cell stage with miR-430 RNA duplex display rescued cell morphology and organization. (G) Scatter labeling strategy. 1-2 blastomeres of 16-cell embryos were injected with GFP mRNA. |
|
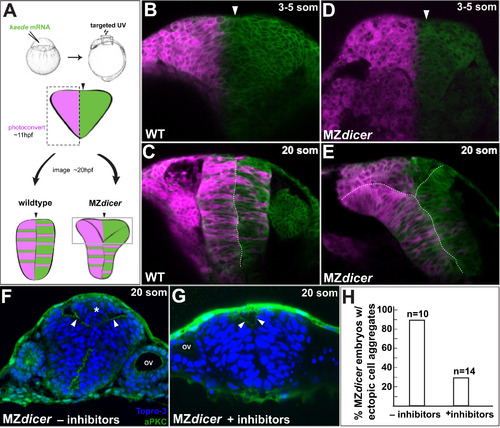
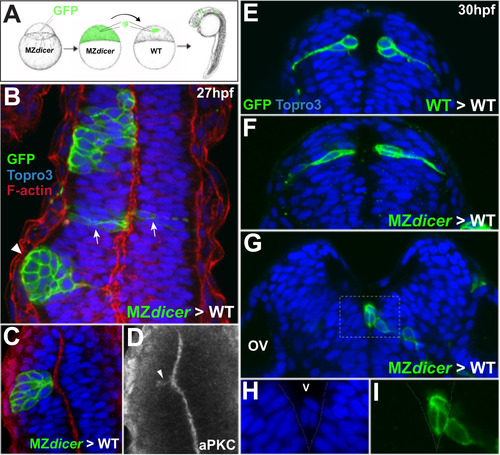
MZdicer neural progenitors in dorsal regions fail to cross midline. (A) Lineage tracing after unilateral Kaede photoconversion at 11 hpf. (B–E) Kaede lineage tracing after photoconversion (3-5 somites; B and D) and ~8 h later (20 somites; C and E). (B–C) Wildtype neural progenitors undergo bilateral distribution. (D–E) In contrast, MZdicer neural progenitors retain unilateral distribution in dorsal regions of the neural anlage. Arrowhead demarcates the presumptive neural midline. (F–H) Cell cycle inhibition reduces MZdicer ectopic neural cell accumulation. Asterisk indicates presence of ectopic cell aggregate. Arrowheads indicate apical membrane. OV, otic vesicle. |
|
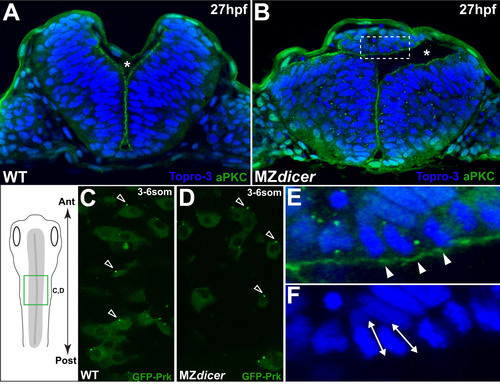
Ectopic MZdicer neural progenitors retain apicobasal and PCP polarity. (A and B) Transverse sections at 27hpf. Topro3, nuclear stain. At this stage, lumen inflation has initiated. Asterisk indicates luminal space. (B) MZdicer embryo with a dorsal ectopic cell aggregate. (E) Inset of panel B; arrowheads indicate aPKC (green) localization to apical membrane. (F) Inset of panel B; double arrows indicate elongated nuclear shape characteristic of polarized epithelial cells. (C and D) Dorsal view (green box in schematic) of GFP-Prickle scatter labeling. GFP-Prk localizes to the anterior edge (arrowheads) of (C) wildtype neural cells (71/94; 76%) and (D) MZdicer mutant cells (39/49; 80%). Anterior to top. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) |
|
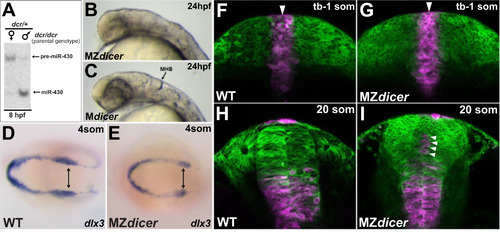
miR-430 activity is required post-gastrulation within proximal neural progenitors (A) Northern blot analysis of miR-430 expression: lane 1, embryos derived from dcr/dcr female germline (maternally deficient) X dcr/+ male; lane 2, embryos derived from dcr/dcr male germline X dcr/+ female (maternally sufficient). Embryos deficient for maternal dicer function do not generate significant levels of mature miR-430 until after 8hpf. (B) MZdicer embryo (maternally and zygotically deficient) fails to generate contiguous neural lumen and ventricular organization. (C) Mdicer embryo (maternally deficient) undergoes ventricle formation, suggesting that zygotic Dicer contribution (and delayed miR-430 production) is sufficient for neural tube generation. (D and E) In situ analysis of dlx3 expression, marking the lateral edges of neural keel. (F–I) Kaede lineage tracing after photoconversion at dorsal midline (tailbud-1somite stage; F, G) and ~10 hours later (20 somite stage; H, I). Arrowheads demarcate dorsal midline. As ingression proceeds, proximal progenitors adopt ventral positions in neural rod. In MZdicer embryos, a subset of proximal progenitors fails to ingress ventrally (arrowheads in panel I). |
|
Transplanted MZdicer cells display reduced ability to integrate into wildtype neuroepithelium. (A) At midblastula stage, ~50 cells (WT or MZdicer) were transplanted into equivalently staged WT embryos. (B) MZdicer clones that display unilateral distribution (asterisk) and/or segregation away from wildtype epithelium (arrowhead). Arrows demarcate a MZdicer clone that displays normal cell shape and bilateral distribution. (C and D) Unilateral MZdicer clone that is organized around ectopic apical membrane (localized aPKC signal; arrowhead in panel D). (E and F) Transverse views of wildtype clone (E) and MZdicer clone (F) displaying epithelial integration and bilateral distribution. (G) Transverse view of MZdicer progeny cells excluded from the neuroepithelium. (H and I) Inset of panel F. |
|
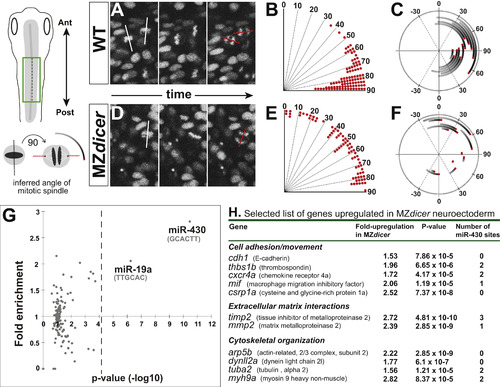
MZdicer neural progenitors undergo misoriented neural rod cell divisions. (A and D) Time-lapse recording from dorsal view (green box in schematic). Histone2A-cherry localizes to chromosomes and enables measurement of mitotic orientation (red lines) at anaphase. White line indicates orientation at initiation of mitosis. (B and E) Quantification of mitotic orientation at anaphase relative to anteroposterior axis (0 degrees) in (B) wildtype (96 cells; 4 embryos) and (E) MZdicer embryos (72 cells; 5 embryos). (C and F) Representation of mitotic spinde rotation for randomly chosen (C) wildtype (n=12) and (F) MZdicer (n=12) progenitors. Inferred spindle orientation (relative to anteroposterior axis) was measured at prophase (start of line) and then again at anaphase (red). (G) miRNA target site (7-mer sites) enrichment in 3′UTRs of upregulated genes (>1.5 fold) in MZdicer neuroectodermal cells. The enrichment of 135 target sites compared to a control set is plotted. The p-value cutoff after multiple test correction is shown as a dashed line. (H) Selected candidate list of upregulated transcripts in MZdicer mutant. Fold-change upregulation and p-value derived from published microarray data ( Shkumatava et al., 2009). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) |
|
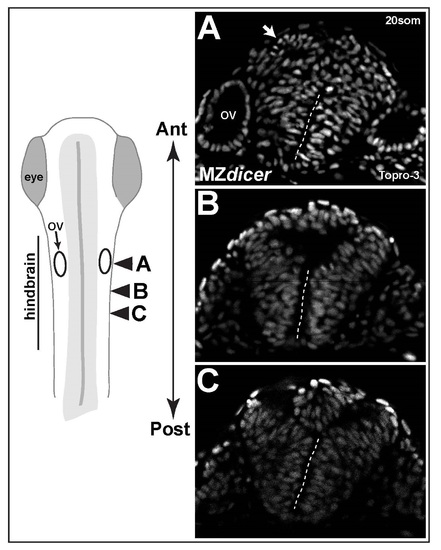
Variation in neural midline defects within same embryo. (A-C) Successive transvere sections along anteroposterior axis of hindbrain reveal variation in dorsal defects. Arrow in panel A demaractes a ectopic rosette of neural progenitors. Dashed line demarcates intact ventral midline. OV; otic vesicle. |
|
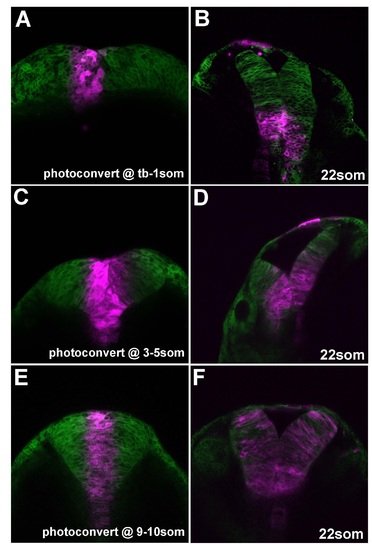
Early proximal progenitors adopt ventral positions in neural tube. Neural progenitors located at the midline were photoconverted at the stages indicated (A, C, E) and re-imaged at later timepoint (B, D, F). |
|
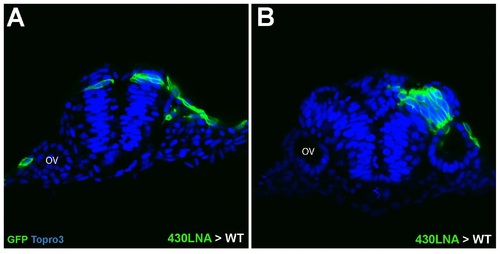
miR-430 KD cells transplanted into wildtype hosts. (A) Bilateral distribution of isolated miR-430 LNA-injected cells suggests successful crossing division in wildtype environment. (B) A large clone of miR-430 KD cells displaying unilateral distribution and associated nonautonomous defects in midline organization. |
|
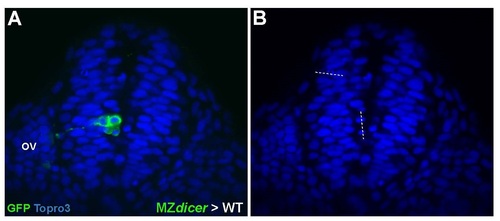
Unilateral distribution of MZdicer cells. (A) MZdicer clone shows failure to deposit daughter cell within contralteral epithelium (B) Mitotic orientation of MZdicer cell division is oriented parallel to neural midline. |
Reprinted from Developmental Biology, 409(2), Takacs, C.M., Giraldez, A.J., miR-430 regulates oriented cell division during neural tube development in zebrafish, 442-50, Copyright (2016) with permission from Elsevier. Full text @ Dev. Biol.