- Title
-
Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance
- Authors
- Cruz, I.A., Kappedal, R., Mackenzie, S.M., Hailey, D.W., Hoffman, T.L., Schilling, T.F., Raible, D.W.
- Source
- Full text @ Dev. Biol.
|
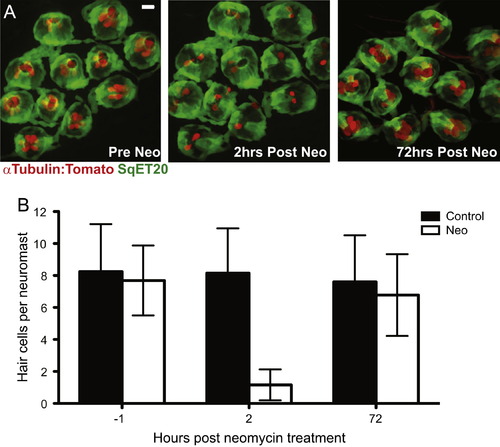
Adult zebrafish lateral line hair cell regeneration after neomycin-induced ablation. Confocal z-stack maximum projection images of a peduncle stitch (11 neuromasts) of an adult sqet20Et;Tg(Ca-tuba1a:tdTomato) zebrafish with hair cells labeled with tdTomato (red) and a subset of support cells labeled with GFP (green). (A) Adult zebrafish peduncle neuromasts (left panel) were treated with 400 µM neomycin for 1 h (middle panel) and then allowed to recover for 72 h to assess hair cell regeneration (right panel). (B) Results are graphed as average number of hair cells per neuromast (±s.d.) for each treatment group. N=4 adult zebrafish per group, 40+ neuromasts per group. Scale bar, 10 µm. |
|
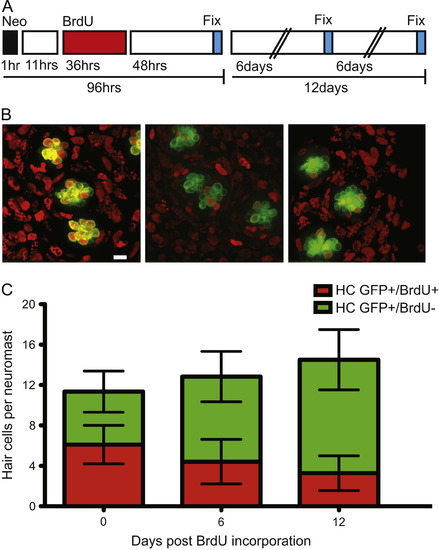
In adult zebrafish, BrdU labeled lateral line hair cells are lost over time under ambient conditions. (A) Schematic illustrating the experimental protocol: fish were treated with 400 µM neomycin for 1 h, then allowed to recover for 11 h, followed by a 36 h 5 mM BrdU incubation period, and finally fixed and stained at the indicated time-points. (B) Representative confocal z-stack maximum intensity projection images of neuromasts with hair cells labeled green with GFP and BrdU positive cell nuclei marked red. BrdU+ hair cells 0 days (left panel), 6 days (middle panel), and 12 days (right panel) after hair cell regeneration. Scale bar, 10 µM (C) Graph illustrating stacked averages (±s.d.) of GFP+ only hair cells (green) and GFP+ hair cells co-labeled with BrdU (red). N=2–3 adult zebrafish per condition, 30+ neuromasts per group. |
|
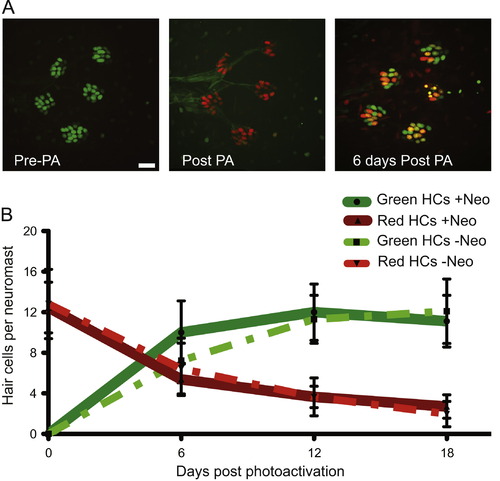
Hair cells are continuously lost and replaced in adult zebrafish lateral line. (A) Representative z-stack confocal maximum projection images of an adult Tg(myo6b:NLS-Eos) peduncle stitch (5 neuromasts shown) before photo-activation, or PA (left panel), immediately after PA (middle panel), and 6 days after PA (right panel). Because Eos is under the control of the myo6b promoter, green Eos is continuously produced therefore hair cells that were present at the time of photo-activation will have both green and red Eos present producing orange/yellow colored nuclei. Scale bar, 10 µM. (B) Fish were treated without or with neomycin to synchronize hair cell age through hair cell regeneration; then nuclear localized Eos was laser activated in all hair cells and followed over time. The results are graphed for the two pulse-chase hair cell turnover experiments, one without hair cell age synchronization and the other with. Dotted lines represent averages per neuromast (±s.d.) of hair cells that were present at the time of Eos protein activation (red) or hair cells generated post-Eos laser activation (green) without neomycin treatment. N=3 adult zebrafish, 20 neuromasts. Solid lines represent averages per neuromast (±s.d.) of hair cells that were present at the time of Eos protein activation (red) or hair cells generated post-Eos activation (green) with neomycin-induced hair cell age synchronization. N=3 adult zebrafish, 17 neuromasts. |
|
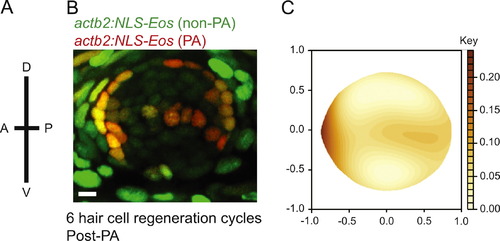
Label retaining support cells are localized to the anterior region of caudal fin neuromasts. (A) Schematic illustrating the orientation of neuromasts in the caudal fin that were analyzed; applies to panels B and C (B) representative z-stack confocal maximum projection image of an adult Tg(actb2:NLS-Eos) neuromast following entire neuromast Eos photoactivation (PA) and 6 sequential hair cell regeneration cycles. Scale bar, 10 µM. (C) Diagram illustrating the distribution of label retaining cells using the red fluorescence intensity smooth median regression of 23 overlaid neuromasts. N=4 adult zebrafish. |
|
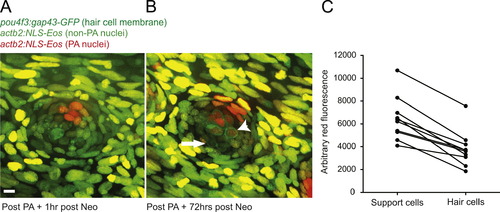
Selective support cell Eos activation shows non-equitable hair cell contribution during hair cell regeneration. (A) Selective support cells in adult Tg(pou4f3:gap43-GFP;actb2:NLS-Eos) caudal neuromasts were activated after neomycin treatment. Arrow indicates surviving hair cell without red nuclei. (B) Hair cell production was traced from labeled support cells during hair cell regeneration. Arrowhead illustrates hair cell with red nuclei produced from support cell with activated red Eos while the arrow illustrates a hair cell with no activated Eos protein. Scale bar, 10 µm. (C) The average red fluorescence of locally activated support cells and regenerated hair cells of 10 different neuromasts following hair cell regeneration. |
Reprinted from Developmental Biology, 402(2), Cruz, I.A., Kappedal, R., Mackenzie, S.M., Hailey, D.W., Hoffman, T.L., Schilling, T.F., Raible, D.W., Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance, 229-38, Copyright (2015) with permission from Elsevier. Full text @ Dev. Biol.