- Title
-
Mutual antagonism of the paired-type homeobox genes, vsx2 and dmbx1, regulates retinal progenitor cell cycle exit upstream of ccnd1 expression
- Authors
- Wong, L., Power, N., Miles, A., Tropepe, V.
- Source
- Full text @ Dev. Biol.
|
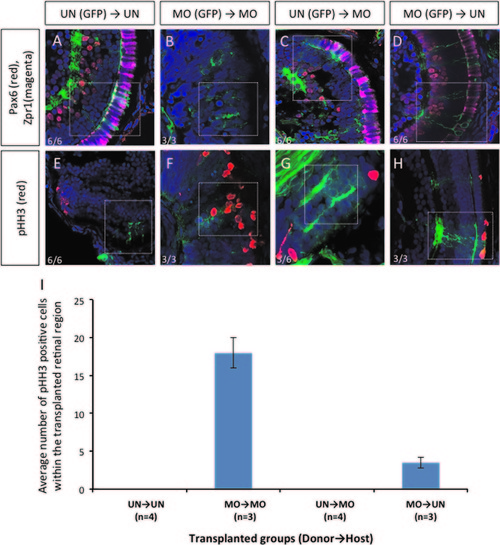
Transplantation experiments demonstrated that dmbx1 functions cell autonomously in the developing retina. Coronal sections of 72 hpf host embryos transplanted with β-actin:GFP positive donor cells at blastula stage. Cells from un-injected (UN) or morpholino-injected (MO) Tg(β-actin:GFP) donor embryos were transplanted to either UN- or MO host embryos. Animals with mosaic retinae were selected for further analysis using immunohistochemistry: Pax6 (RGCs and amacrine cells) and Zpr1 (cone photoreceptor) (A–D, n=6 in each UN donor group and n=3 in each MO donor group); and phospho-Histone H3 [pHH3] (E–H, n=6 in each UN donor group and n=3 in each MO donor group). Images were captured using a confocal microscope without any maximum projection. White dotted boxes highlight areas where GFP+ donor cell localization in the host retina. Numbers of pHH3+ cells within those transplanted cells in the retina of the host embryos are summarized in graph (I). |
|
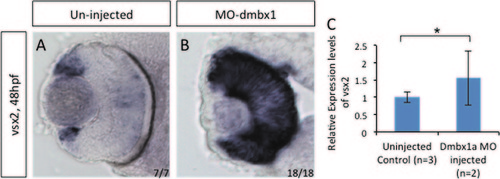
vsx2 gene expression in MO1a+MO1b embryos. In situ hybridization of vsx2 gene expression at 48 hpf (A and B). RT-qPCR was used to quantify RNA from three pools of 50 embryos collected from uninjected and morpholino injected embryos (C). There was a significant increase in vsx2 expression using Student′s t-test (p<0.05). Standard deviation was calculated from three replicate experiments. |
|
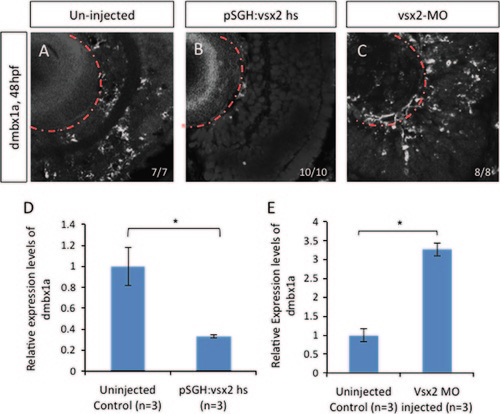
Expression pattern of dmbx1a in embryos with varying vsx2 levels. In situ hybridization of dmbx1a gene expression at 48 hpf in uninjected embryos (A), induced pSGH:vsx2 injected embryos (B) and vsx2 morphants (C). RT-qPCR was used to quantify RNA from three pools of 50 embryos collected from uninjected, pSGH:vsx2 injected, and vsx2 morpholino injected embryos (D and E). There was a significant decrease in dmbx1a expression at 72 hpf when vsx2 is over expressed. In contrast, there was a significant increase at 48 hpf in dmbx1a expression when vsx2 was knocked down. Standard deviation was calculated from three replicate experiments. Statistical significance was accomplished using Student′s t-test (p<0.05). |
|
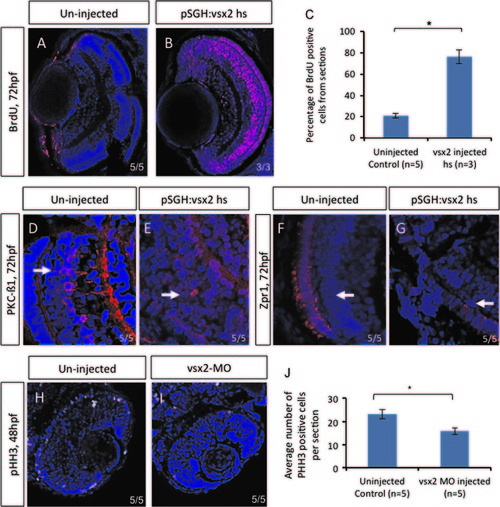
Over-expressing vsx2 embryos resulted in an increase of BrdU positive cells. Immunohistochemistry was performed on sections from uninjected (A) and pSGH:vsx2 injected (B) embryos that were heat shocked at 48 hpf and assessed at 72 hpf. There was a significant increase in the percentage of BrdU positive cells per section compared to uninjected (C). Standard deviation was calculated from four replicate experiments. These retinas also displayed delayed neuroretinal differentiation, as seen with PKC-B1 (D and E) and Zpr1 (F and G). Immunohistochemistry on sections from vsx2 morphants at 48 hpf showed a significant decrease in pHH3 cells (H–J). Statistical significance was assessed using Student′s t-test (p<0.05). PHENOTYPE:
|
|
Retinal progenitor cells undergo premature cell cycle exit in dmbx1-overexpressing embryos. Dmbx1a and dmbx1b mRNAs were co-injected in the embryos at the 1-cell stage to test whether Dmbx1 can sufficiently promote cell cycle exit in retinal progenitor cells. Indeed, when dmbx1 genes are misexpressed, these embryos (B and D) have reduced amount of proliferating cells in the retina when compared to un-injected embryos (A and C) as indicated by BrdU (A and B) and pHH3 (C–E) staining at 1 dpf. These progenitor cells are forced to become post-mitotic prematurely. Un-injected and misexpressing embryos were assessed for Zn5 (F and G), GS (H and I), and Zpr1 (J and K) at 72 hpf. There was a noticeable expansion of Zn5 cells in the ectopic dmbx1 embryos. EXPRESSION / LABELING:
|
|
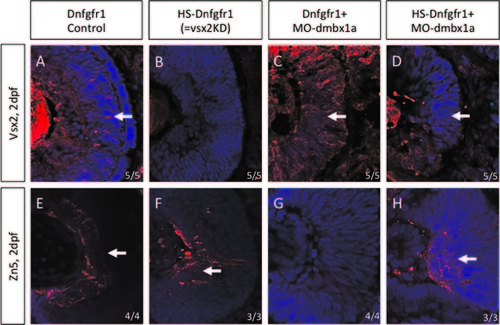
The Tg(hsp70l:dnfgfr1-EGFP) transgenic line provides a temporal model for loss of vsx2. Immunohistochemistry was performed on retinal sections of Dnfgfr1- no heat shock, Dnfgfr1-heat shock, Dnfgfr1+MO1a-no heat shock, and Dnfgfr1+MO1a-heat shock at 48 hpf. The markers used are Vsx2 (A–D) and Zn5 (E–H). Vsx2 expression was not completely rescued when both dmbx1 and FGF signaling was knocked down but differentiation of Zn5 positive ganglion cells was partially rescued. |
|
Whole mount in situ hybridization of cyclinD1 (ccnd1) expression changes when dmbx1 is perturbed. Between 48 and 72 hpf, ccnd1 expression was detected in the CMZ region in control embryos (A and D) but in dmbx1 MO-injected embryos, expression had expanded from the CMZ to the central retina (B and E). When dmbx1 was ectopically expressed, there was a strong reduction of ccnd1 expression around the CMZ region (C and F). EXPRESSION / LABELING:
PHENOTYPE:
|
|
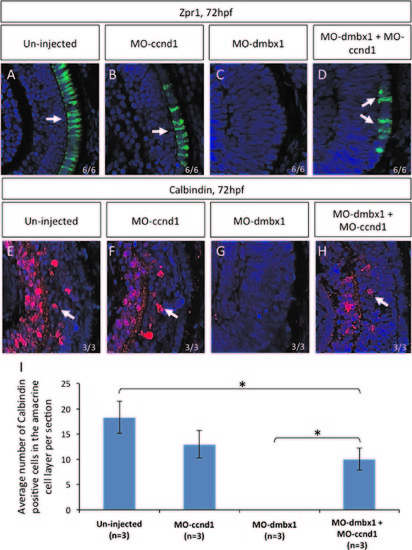
Repression of cyclinD1 partially rescued dmbx1 morphants phenotype. Coronal cryosections of 72 hpf retina (A–H). Retinal differentiation in the central retina can be seen using immunohistochemistry with Zpr1 (labels photoreceptors) and Calbindin (labels retinal ganglion+amacrine cells) markers. Both un-injected (A and E) and MOccnd1 (B and F) embryos express differentiation retinal markers. No differentiation markers are shown in dmbx1 morphants (MO1a+MO1b) (C and G). When dmbx1 morphants are knocked down with cyclin D1 morpholino (MO1a+MO1b+MOccnd1) (D and H), cells in the central retina are able to differentiate into specific cell types normally. Arrows point to the differentiation markers that are present in the retina. Numbers of Calbindin-positive cells observed in the amacrine cell layer per retinal section from each injection groups are summarized in graph (I), asterisks represent significant difference in the number of Calbindin-positive cells between two injection groups (p<0.05). EXPRESSION / LABELING:
PHENOTYPE:
|
|
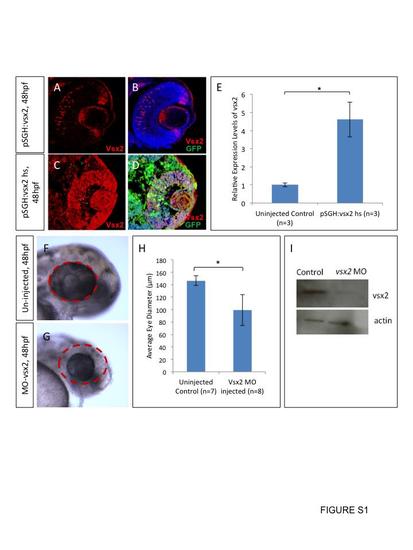
Over expression of vsx2 and knock down of vsx2 in wild type embryos. Embryos were injected with pSGH:vsx2, heat shocked at 24 hpf and fixed at 48 hpf. Cryosections of 20 µm were labeled with anti-vsx2 and anti-GFP (A–D) (n=5). Transcript levels of vsx2 were also measured using RT-qPCR (D). There was a significant increase in vsx2 gene expression at 72 hpf (D). Three pools of RNA from 50 embryos were collected for RT-qPCR. Standard deviation was calculated from three replicate experiments. Translational blocking morpholinos were injected into wild type embryos and these morphants displayed microphthalmia with a significant decrease in eye diameter (F–H). Western blot of protein from the translational blocking morpholino injected embryos displayed lower vsx2 protein (I). Standard deviation was calculated from five replicate experiments. Statistical significance was assessed using Student′s t-test (p<0.05). |
|
Un-injected heat-shocked embryos do not display growth defects. Un-injected embryos (n>20 for each group) were heat-shocked at either 24 hpf or 48 hpf for 60 min at 37 °C followed by 60 min at 28.5 °C and this was repeated for a total of three times. Embryos were then allowed to develop at 28.5 °C until the stage of analysis. Controls were not heat-shocked, but were derived from the same clutches and raised at 28.5 °C. |
|
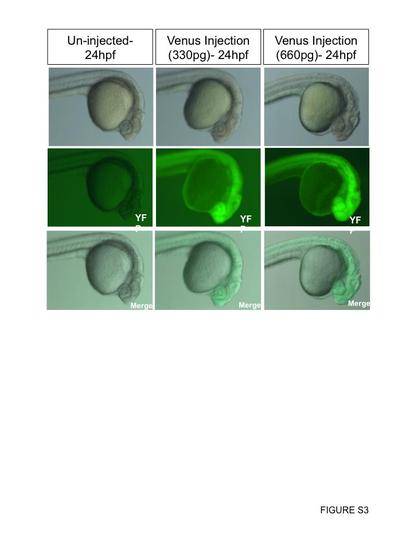
Reporter mRNA injections do not affect development. Venus (variant of YFP) mRNA was injected into the yolk of embryos at the one-cell stage. The injection concentration used was 167 ng/uL. About 2 nL was injected into one group, which is equivalent to about ~330 ng (n=47). About 4 nL (double the amount) was injected into a second group, which is equivalent to ~660 ng (n=34). All embryos injected fluoresce GFP. Representative images were taken at 24 hpf. |
|
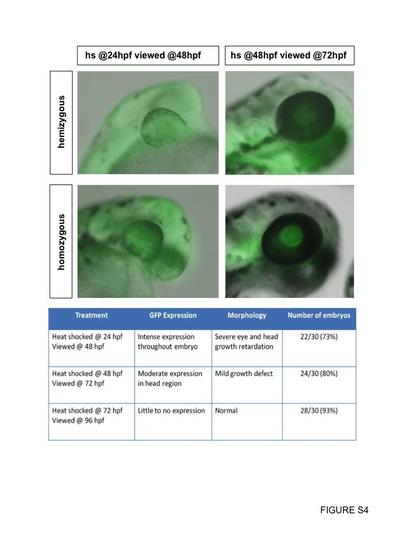
Heat-shock of hemizygous or homozygous Dnfgfr1 transgenics. Transgenic embryos at either 24 hpf or 48 hpf were heat-shocked once for 60 min at 37 °C followed by normal rearing at 28.5 °C until the desired stage. Our analyses suggested that the intensity of GFP expression and the severity of the loss of function phenotype varied according to the developmental stage at which the heat-shock was administered rather than transgene copy number. For this reason, our analyses included mixed populations of hemizygous and homozygous transgenic embryos (including the data shown in the chart). |
|
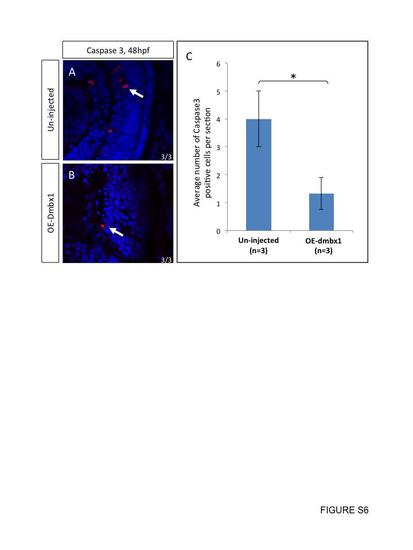
No increase in cell death was observed in dmbx1-overexpressing embryos. Retinal neurons that prematurely exited the cell cycle do not appear to induce cell death. At 2 dpf, un-injected controls (A) and embryos ectopically expressed dmbx1 (B) were sectioned and immunolabelled with Caspase 3 antibody (n=3 in each group). Number of Caspase 3 positive cells per section was summarized in the graph (C). White arrows point to positive-staining cells. |
|
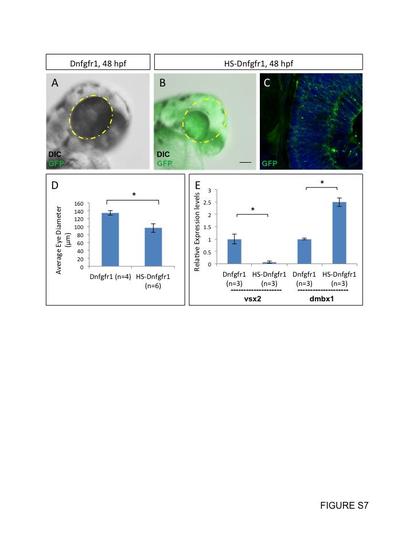
Heat induction of Tg(hsp70l:dnfgfr1-EGFP) decreases vsx2 gene expression. Heat induction of the transgenic line, Tg(hsp70l:dnfgfr1-EGFP), at 48 hpf resulted in microphthalmia, with uniform gfp expression throughout the retina, which had a significantly reduced eye diameter (A–D). Standard deviation was calculated from six replicate experiments. Gene expression levels of vsx2 significantly decreased upon heat induction and gene expression levels of dmbx1a significantly increased (E). RNA from three pools of 50 embryos was quantified using RT-qPCR and standard deviation was calculated from three replicate experiments. Statistical significance was measured using Student’s t-test (p<0.05). |
|
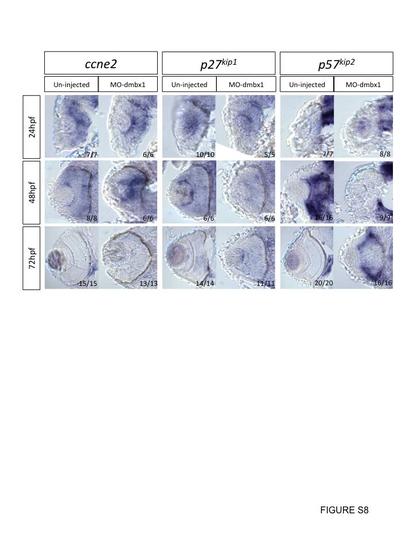
Cryosection on whole mount in situ hybridization embryos with various cell cycle markers at 24–72 hpf. All sections are in coronal view, development stages and probe used in each case is listed on the left and on the top respectively. Uninjected embryos and dmbx1 morphant coronal retinal sections of ccne2, p27kip1, and p57kip2 are all taken from whole mount in situ embryos. Expression of p57kip2 expression came up slower in dmbx1-deficiency embryos when compare to un-injected embryos, while expression of ccne2 and p27kip1 remained the same in both control and dmbx1 morphant embryos. |
|
Mismatch control MO does not rescue dmbx1 morphant phenotype. When dmbx1 morphants are knocked down with ccnd1 MO, Calbindin+ cells in the central retina are able to differentiate. In contrast, in the presence of a control ccnd1 mMO, dmbx1 morphant retinas do not show Calbindin+ amacrine cell differentiation. On average, 2 central sections were analyzed from n=3 embryos per treatment group. |
Reprinted from Developmental Biology, 402(2), Wong, L., Power, N., Miles, A., Tropepe, V., Mutual antagonism of the paired-type homeobox genes, vsx2 and dmbx1, regulates retinal progenitor cell cycle exit upstream of ccnd1 expression, 216-28, Copyright (2015) with permission from Elsevier. Full text @ Dev. Biol.