- Title
-
Intrinsic and extrinsic innervation of the heart in zebrafish (Danio rerio)
- Authors
- Stoyek, M.R., Croll, R.P., Smith, F.M.
- Source
- Full text @ J. Comp. Neurol.
|
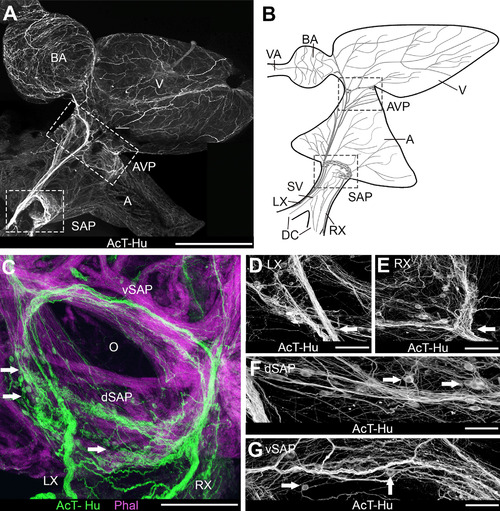
Organization of intracardiac nervous system demonstrated with acetylated tubulin (AcT) and human neuronal protein (Hu) immunohistochemistry. A,B: Whole mount of heart (A) and schematic (B) show an overview of the chambers of the heart and the major elements of cardiac innervation. Schematic represents the heart as it would appear after removal from the body for making whole-mount cardiac preparations. Blood passes serially from the paired ducts of Cuvier (DC) into the sinus venosus (SV), through the sinoatrial valves and into the atrium (A), then into the ventricle (V), the bulbus arteriosus (BA), and the ventral aorta (VA) to the gills. The lower boxed areas in A and B are the region containing the sinoatrial valve, where the sinoatrial plexus (SAP) was located. In A the upper box indicates the region of the atrioventricular plexus (AVP) at the atrioventricular junction. RX, LX, right and left vagosympathetic trunks. C-G: Details of innervation in the region of the sinoatrial valve (O: valve ostium). C: Cardiac myocytes were labeled with phalloidin (Phal); arrows indicate neuronal somata. dSAP, vSAP: dorsal and ventral regions, respectively, of SAP. D,E: Details of SAP where LX and RX, respectively (arrows), enter the plexus. Somata were clustered into ganglia in each region. F,G: Dorsal and ventral SAP, between the areas shown in D,E. Arrows indicate somata. Scale bars = 1 mm in A; 250 µm for B; 100 µm in C,D; 75 µm in E-G. |
|
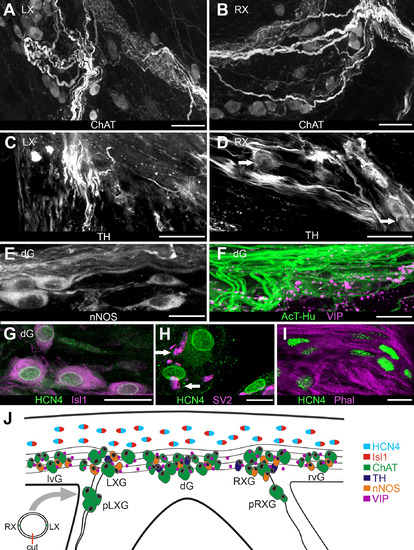
Localization of neurotransmitter-specific elements and presumptive pacemaker tissue in the sinoatrial valve region. A,B: Choline acetyltransferase (ChAT)-labeled axons and somata at junctions of left (A) and right (B) vagosympathetic nerves with SAP. C,D: Tyrosine hydroxylase (TH)-positive axons in same regions as shown in A,B. Arrows in D indicate TH-positive somata. E: Antineuronal nitric oxide synthase (nNOS)-labeled axons and somata in the dorsal SAP; somata were located in a ganglion here (dG). F: Vasoactive intestinal polypeptide (VIP)-positive axons and terminals were located in dorsal SAP. G: Putative pacemaker cells in base of dorsal sinoatrial valve were double labeled with antibodies against hyperpolarization-activated cyclic-nucleotide gated ion channels (HCN4) and Islet-1 (Isl1). H: Antisynaptic vesicle 2 (SV2) labeled axon terminals (arrows) proximal to HCN4-positive cells. I: HCN4-positive cells embedded in myocytes (Phal) in ventral valve leaflet. J: Schematic of SAP region oriented to show locations of elements expressing specific phenotypes (color-coding key on right side). To obtain this tissue orientation, a transverse cut was made through the ventral sinoatrial valve leaflet between junctions of the left and right vagosympathetic trunks with the SAP (diagram, lower left); tissue was then flattened and spread. Atrium is at upper edge of the panel. Neurons were clustered into ganglia (G) by region; from left to right these are left ventral (lvG), left vagal (LXG); dorsal (dG), right vagal (RXG); right ventral (rvG). In all preparations there were varying numbers of neurons in small ganglia associated with the vagosympathetic trunks near the SAP (proximal left and right vagal [pLXG, pRXG]). Putative pacemaker cells were doubly color coded to indicate combined HCN4-Isl1 labeling. Scale bars = 100 µm in A-D; 50 µm in E,F; 5 µm in G; 2 µm in H; 10 µm in I. |
|
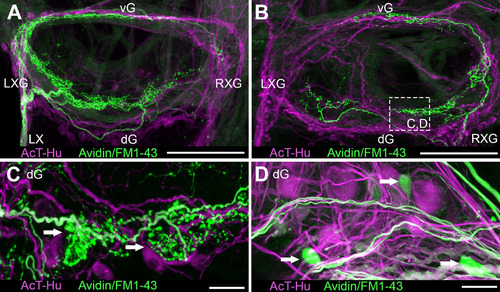
Examples of intracardiac tracing of extrinsic vagosympathetic inputs to SAP by neurobiotin and FM1–43X application to proximal stumps of nerve trunks (neurobiotin visualized with avidin secondary). A,B: Axons and terminals within SAP shown after tracer application to left (A) and right (B) extrinsic nerves; both tissue samples were double labeled with AcT-Hu. C: Details of terminals associated with extrinsic axons in neuropil near junction of right vagosympathetic trunk with SAP (boxed area in B). Some labeled terminals appeared to be apposed to AcT-Hu-positive neuronal somata (arrows). D: Arrows indicate putative somata and associated axons labeled with neurobiotin, among larger AcT-Hu-positive somata and neuropil. Scale bars = 100 µm in A,B; 50 µm in C; 20 µm in D. |
|
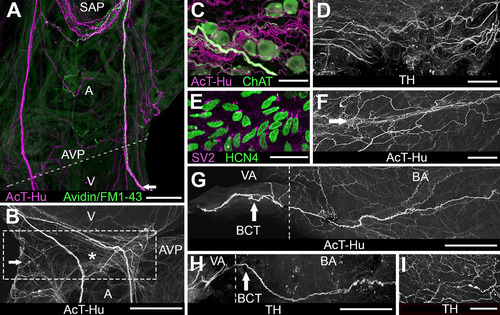
Innervation of atrioventricular region and outflow tract. A: Atrium and ventricle double labeled with AcT-Hu and neurotracer (avidin/FM1–43X, applied to right vagosympathetic trunk) show nerve trunks arising from SAP (upper part of panel) and projecting toward atrioventricular junction (dashed line; AVP: site of atrioventricular plexus). Some axons continued from AVP into ventricle (V) at lower edge of the panel. Presence of neurotracer indicates that a portion of axons originated extrinsic to the heart. B: Enlargement of region containing AVP (boxed area) from another specimen labeled with AcT-Hu shows the contribution of atrial nerve trunks to plexus; a small ganglion is indicated by the arrow; asterisk marks location of atrioventricular valve. C: Detail of AVP shows cholinergic somata associated with cholinergic and noncholinergic axons in neuropil. D: TH-labeled axons in AVP neuropil. E: HCN4-positive cells located in region of atrioventricular valve (asterisk in B). F: AcT-Hu-positive axons within atrioventricular valve leaflets (near asterisk). G: AcT-Hu-labeled innervation in walls of outflow tract (BA, bulbus arteriosus; VA, ventral aorta). Dashed line marks the border between VA and BA. A prominent nerve trunk (branchiocardiac trunk [BCT], arrow) coursed cephalocaudally in the wall of the BA toward the ventricle (right edge of panel). H: TH labeled a subset of axons in the BCT (arrow). I: BA wall was innervated with a network of fine TH-positive axons. Scale bars = 500 µm in A; 100 µm in B; 50 µm in C-E,G,I; 75 µm in F,H. |