- Title
-
IFT88 Plays a Cilia- and PCP-Independent Role in Controlling Oriented Cell Divisions during Vertebrate Embryonic Development
- Authors
- Borovina, A., and Ciruna, B.
- Source
- Full text @ Cell Rep.
|
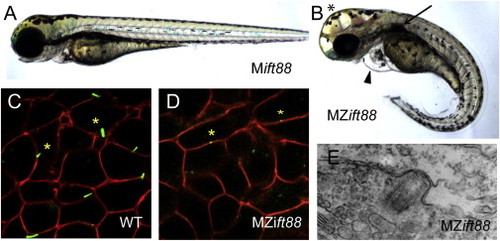
MZift88 Mutants Do Not Form Cilia (A and B) Lateral view of a 3-day-old (A) Mift88 embryo and (B) MZift88 mutant embryo exhibiting a curved body axis, pericardial edema (arrowhead), hydrocephalus (asterisk), and a cystic kidney (arrow). (C and D) Confocal images of (C) WT and (D) MZift88 mesoderm cells, at 9 hpf, expressing Arl13b-GFP (green) and memb-mRFP (red) revealed the presence of cilia on WT cells (asterisks, C). Only small puncta of Arl13b-GFP were visible on MZift88 mutant cells (asterisks, D). (E) Transmission electron microscopy micrograph of basal body docking in the neural tube of a 30hpf MZift88 mutant embryo. PHENOTYPE:
|
|
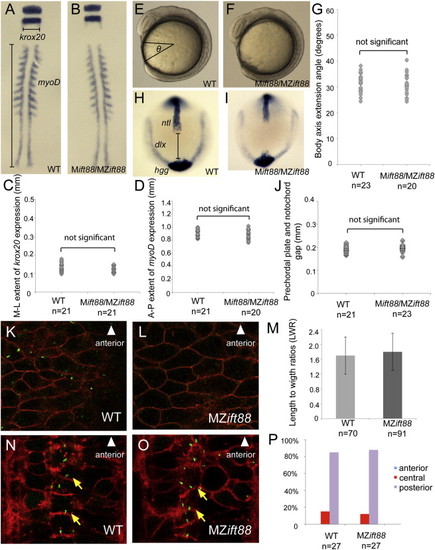
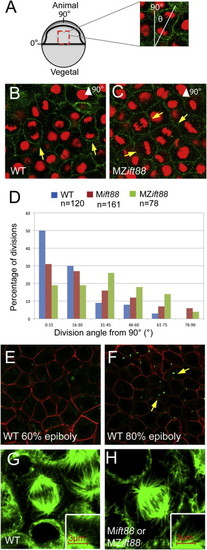
Cilia Are Not Required to Establish PCP (A–D) Whole embryo flat-mount RNA in situ hybridization of krox20 and myoD expression in representative ten-somite-stage WT (A) and Mift88 or MZift88 (B) embryos. Quantification of the mediolateral extent of krox20 (C; p = 0.06) and the anteroposterior extent of myoD expression (D; p = 0.21) demonstrated no significant differences in CE of the body axis between WT (n = 21) and Mift88 or MZift88 mutants (n = 21, krox20; n = 20, myoD). (E–G) Lateral DIC images of eight-somite-stage WT (E) and Mift88 or MZift88 (F) embryos. Quantification of the body axis extension angle (G) in WT (n = 23) and Mift88 or MZift88 mutants (n = 20) demonstrated no significant differences (p = 0.36). (H–J) Whole-mount RNA in situ hybridization of ntl, hgg1, and dlx expression in representative bud-stage WT (H; n = 21) and Mift88 or MZift88 (I; n = 23) embryos. Quantification of the gap between the prechordal plate and the notochord (J) revealed no significant differences (p = 0.1). (K–M) Confocal micrographs of WT (K) and MZift88 (L) dorsal ectoderm cells labeled with memb-mRFP (red) and Arl13b-GFP (green). (M) Graph of LWRs for WT (n = 70; 2 embryos) and MZift88 mutant (n = 91; 3 embryos) cells revealed no statistical differences (p = 0.25). Error bars in (M) represent SD. (N–P) Coronal section through the floor plate of 28–32 hpf WT (N) and MZift88 mutant (O) embryos expressing memb-mRFP (red) and Centrin-GFP (green) demonstrating basal body docking at the posterior apical surface (yellow arrows). (P) Quantification of WT (n = 27) or MZift88 (n = 27) cells displaying anterior (blue), central (red), and posterior (purple) position of basal bodies at the apical surface of floor-plate cells. Statistical significance for all experiments was determined using a Student’s t test and alpha was set as 0.05. EXPRESSION / LABELING:
|
|
Cilia Are Not Required to Establish PCP (A–D) Whole embryo flat-mount RNA in situ hybridization of krox20 and myoD expression in representative ten-somite-stage WT (A) and Mift88 or MZift88 (B) embryos. Quantification of the mediolateral extent of krox20 (C; p = 0.06) and the anteroposterior extent of myoD expression (D; p = 0.21) demonstrated no significant differences in CE of the body axis between WT (n = 21) and Mift88 or MZift88 mutants (n = 21, krox20; n = 20, myoD). (E–G) Lateral DIC images of eight-somite-stage WT (E) and Mift88 or MZift88 (F) embryos. Quantification of the body axis extension angle (G) in WT (n = 23) and Mift88 or MZift88 mutants (n = 20) demonstrated no significant differences (p = 0.36). (H–J) Whole-mount RNA in situ hybridization of ntl, hgg1, and dlx expression in representative bud-stage WT (H; n = 21) and Mift88 or MZift88 (I; n = 23) embryos. Quantification of the gap between the prechordal plate and the notochord (J) revealed no significant differences (p = 0.1). (K–M) Confocal micrographs of WT (K) and MZift88 (L) dorsal ectoderm cells labeled with memb-mRFP (red) and Arl13b-GFP (green). (M) Graph of LWRs for WT (n = 70; 2 embryos) and MZift88 mutant (n = 91; 3 embryos) cells revealed no statistical differences (p = 0.25). Error bars in (M) represent SD. (N–P) Coronal section through the floor plate of 28–32 hpf WT (N) and MZift88 mutant (O) embryos expressing memb-mRFP (red) and Centrin-GFP (green) demonstrating basal body docking at the posterior apical surface (yellow arrows). (P) Quantification of WT (n = 27) or MZift88 (n = 27) cells displaying anterior (blue), central (red), and posterior (purple) position of basal bodies at the apical surface of floor-plate cells. Statistical significance for all experiments was determined using a Student’s t test and alpha was set as 0.05. PHENOTYPE:
|
|
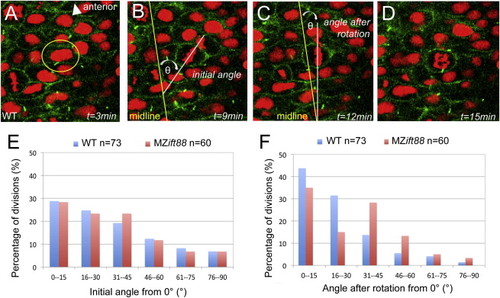
IFT88 Is Required for PCP-Independent OCD at Neurulation (A–D) Confocal images of neural progenitor cell division in a six-somite-stage (12 hpf) WT embryo expressing Arl13b-GFP (green; cell membranes) and histone 2B-Cherry (red; histones). In these cells, the initial orientation of the metaphase plate is realigned to be parallel to the midline so that the subsequent division plane occurs across the midline. Images depict a cell that is about to undergo cell division (A), the initial orientation of the metaphase plate (B), metaphase plate orientation following spindle rotation (C), and the cell in anaphase (D). Elapsed time (t) is indicated in minutes. Spindle orientation was measured as the angle of the metaphase plate relative to the neural midline. (E) Graph of spindle orientations at the onset of metaphase, demonstrating no significant difference (p = 0.830) between WT (blue; n = 73) and MZift88 (red; n = 60) cells. (F) Graph of metaphase plate orientations following spindle rotation. The metaphase plate in WT cells (blue; n = 73) is strongly aligned with the midline, whereas MZift88 cells (red; n = 60) exhibit a statistically significant shift (p = 0.010) to a more randomized orientation. Statistical significance was determined using a Watson-Williams two-sample test and statistical significance was assigned when p < 0.05. PHENOTYPE:
|