- Title
-
Requirement for a uroplakin 3a-like protein in the development of zebrafish pronephric tubule epithelial cell function, morphogenesis, and polarity
- Authors
- Mitra, S., Lukianov, S., Ruiz, W.G., Cianciolo Cosentino, C., Sanker, S., Traub, L.M., Hukriede, N.A., and Apodaca, G.
- Source
- Full text @ PLoS One
|
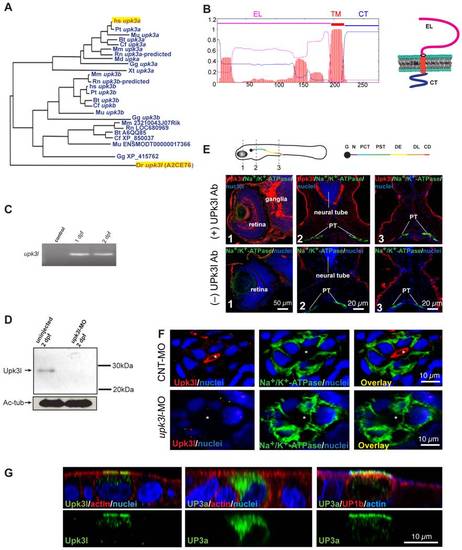
Expression of upk3l in the zebrafish pronephros. (A) Dendrogram of the UP3 family (TreeFam accession TF336628) Legend: Bt, Bos taurus; Cf, Canis familiaris; Dr: Danio rerio; Gg, Gallus gallus; Hs, Homo sapiens; Md, Monodelphis domestica; Mm, Mus musculus; Mu, Macaca mulatta; Pt, Pan troglodytes; Rn, Rattus norvegicus; Xt, Xenopus tropicalis. Upk3l (A2CE76) and human (hsUP3a) are highlighted. (B) TMHMM plot and predicted structure of Upk3l: CT, cytosolic tail; EL, extracellular loop; TM, transmembrane domain. (C) Expression of upk3l message in 1-dpf and 2-dpf embryos as detected by RT-PCR; control: reaction in which the RNA template was substituted with water. (D) Western blot analysis of Upk3l expression in uninjected embryos or those injected with upk3l-MO. Acetylated-tubulin (Ac-tub) was used as a loading control. (E) Localization of Upk3l, Na+/K+-ATPase, and nuclei in cross sections of 2-dpf embryos. The Upk3l primary antibody was added to the samples in the upper row of images, but excluded from those in the lower set. The approximate location of the sections is shown in the cartoon above. Legend: G, glomerulus; N, neck; PCT, proximal convoluted tubule; PST, proximal straight tubule; DE, distal early tubule; DL, distal late tubule; CD, collecting duct. (F) Distribution of Upk3l, Na+/K+-ATPase, and nuclei in the PTs of 2-dpf embryos incubated with control MO (upper panels) or upk3l-MO (lower panels). The lumen is marked with an asterisk. (G) Immunolabeling of MDCK cells transiently transfected with cDNA encoding upk3l, human (h)UP3a alone, or hUP3a and hUP1b. Samples were costained with TRITC-phalloidin to label the cortical actin cytoskeleton and/or TO-PRO-3 to label the nuclei. Images are XZ confocal sections. EXPRESSION / LABELING:
|
|
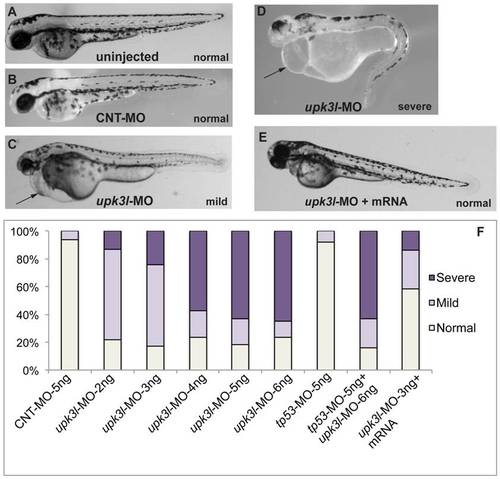
Phenotypes of upk3l morphant embryos. (A–D) Uninjected-, CNT-MO-, or upk3l-MO-injected embryos were examined under a dissecting microscope and photographed. Pericardial edema, when observed, is indicated by a black arrow. (E) Embryo injected with 3 ng upk3l-MO, 100 pg MO-resistant upk3l mRNA, and 50 pg of mCherry mRNA (to mark successful injections). Microinjection of MO-resistant mRNA alone at doses of 50-200 pg did not produce detectable phenotypic abnormalities. (F) Distribution of morphological phenotypes associated with embryos injected with 5 ng CNT-MO, 2-6 ng of upk3l-MO, tp56-MO ±upk3l-MO, or upk3l-MO/upk3l mRNA/mCherry (n = 100). PHENOTYPE:
|
|
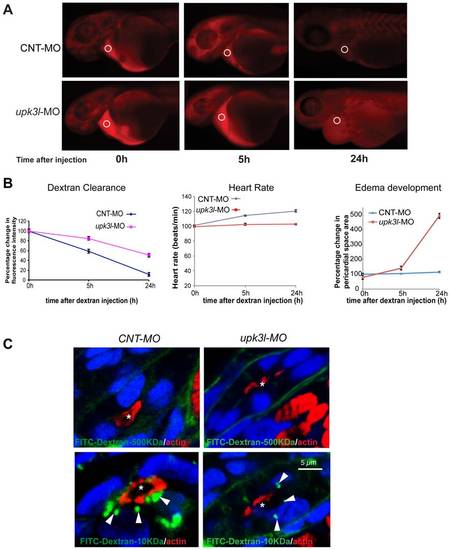
Pronephric clearance, heart rate, and pericardial area in control and morphant embryos. (A) Clearance of 70 kDa-TRITC dextran injected into the common cardinal vein of 54-hpf control (top panel) or morphant (bottom panel) embryos. The fluorescence intensity of the sampled area (circled) was measured in each embryo 0, 5, and 24 h following dextran injection. (B) The heart rate, pericardial area, and the dextran retention was recorded for each embryo and the values relative to t = 0 (post dextran injection) were calculated. Data are reported as mean ± SEM (n = 25). (C) Uptake of 500 kDa or 10 kDa FITC-dextran in control or morphant PT epithelial cells. Tubule lumens are marked by asterisks, while internalized dextran is indicated by arrows. Note the incomplete ring of actin in morphant PT cells. PHENOTYPE:
|
|
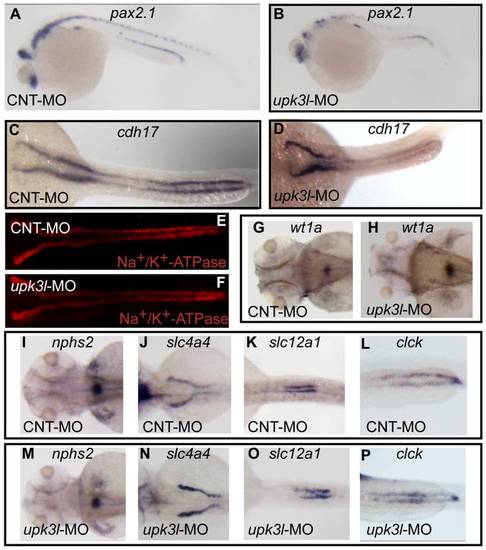
Pronephric morphogenesis, patterning, and segmentation in control and morphant embryos. (A–D) Expression of pax2.1 in 1-dpf embryos or cdh17 in 2-dpf embryos as revealed by in situ hybridization. (E, F) Low magnification overview of Na+/K+-ATPase expression in the PTs of 2-dpf control or morphant embryos. (G–P) Expression of wt1a (G–H), nph2s (I, M), slc4a4 (J,N), slc12a (K, O) or clck (L, P) in control or morphant 3-dpf embryos. EXPRESSION / LABELING:
|
|
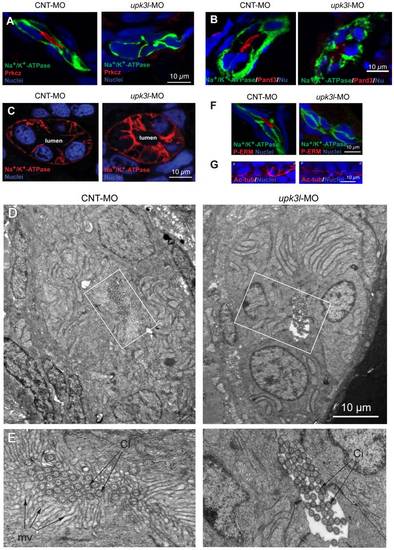
Disruption of epithelial polarity in upk3l morphants. (A–B) Localization of Prkcz (A) or Pard3 (B), Na+/K+-ATPase, and nuclei in cross-sectioned embryos treated with control (CNT-MO) or upk3l MO (upk3l-MO). (C) Distribution of Na+/K+-ATPase and nuclei in 2-dpf control or morphant embryos. (D–E) TEM analysis of the proximal segment of PTs from control or morphant embryos. (D) Cross section of tubule show that it is comprised of 5–6 cells surrounding a central lumen. (E) At higher magnification, control lumens were filled with microvilli (mv) and cilia (ci), whereas microvilli were absent in morphants. (F) Immunolocalization of phosphorylated ERM proteins (P-ERM) in frozen sections of embryos. Sections were co-stained for Na+/K+-ATPase and nuclei. (G) Immunostaining of cilia-associated acetylated-tubulin and nuclei in the PT of laterally oriented embryos. p: proximal tubule, d: distal tubule. |
|
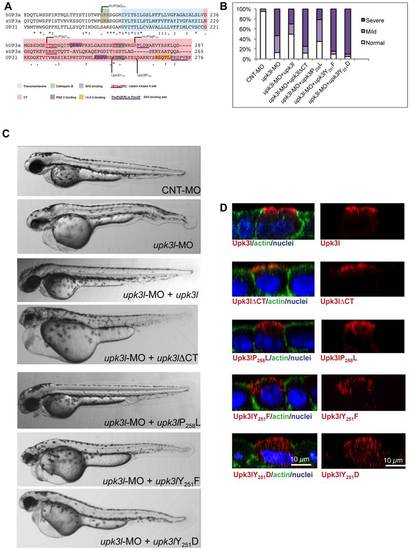
The CT of Upk3l is critical for its function. (A) Clustal W alignment of the TM and CT region of hUP3a, xUP3a and Upk3l. The amino acids that comprise the TM and CT domain are highlighted, and conserved functional motifs and residues are underlined or shaded, respectively. (B) Phenotypes of embryos injected with CNT-MO, upk3l-MO alone, or upk3l-MO co-injected with mCherry mRNA and mRNAs encoding MO-resistant versions of upk3lΔCT, upk3lP258L, upk3lY251F, or upk3lY251D. Microinjection of MO-resistant mRNA alone at the same doses did not produce detectable phenotypic abnormalities. (C) Morphological phenotypes associated with embryos injected with 5 ng CNT-MO (n = 100), 3 ng of upk3l-MO (n = 100), or 3 ng of upk3l-MO and 100 pg of upk3l, upk3lΔCT, upk3lP258L, upk3lY251F, or upk3lY251D mRNA (n≥50). (D) Localization of FLAG-tagged Upk3l, Upk3lΔCT, Upk3lP258L, Upk3lY251F, or Upk3lY251D in MDCK cells co-stained for actin and nuclei. Upk3lY251F and Upk3lY251D showed a predominantly intracellular localization and their low levels of expression necessitated an 1.5-fold increase in photomultiplier voltage above that used for the other samples. |