- Title
-
Soluble THSD7A Is an N-Glycoprotein That Promotes Endothelial Cell Migration and Tube Formation in Angiogenesis
- Authors
- Kuo, M.W., Wang, C.H., Wu, H.C., Chang, S.J., and Chuang, Y.J.
- Source
- Full text @ PLoS One
|
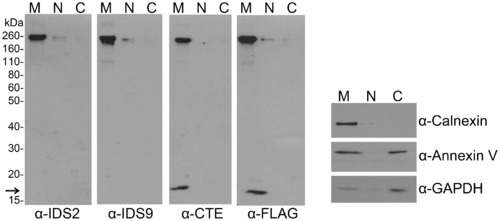
THSD7A is a membrane-associated protein. THSD7A-transfected HEK293T cells were homogenized and the cytosolic (C), nuclear (N) and membrane (M) fractions were prepared as described in the materials and methods. Each fraction was subjected to Western blot with anti-THSD7A (IDS2, IDS9, CTE), anti-FLAG-tag, anti-calnexin (plasma membrane marker), anti-annexin V (cytoplasm and plasma membrane marker) and anti-GAPDH (cytoplasm marker) antibodies. Arrow indicates the carboxyl-terminal fragment of THSD7A. |
|
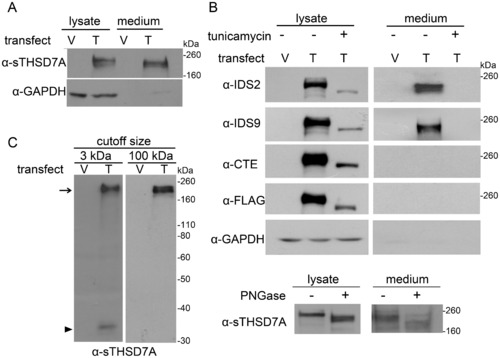
THSD7A is an N-glycoprotein and can release the soluble form into the extracellular environment. A. Empty vector (V)- and THSD7A (T)-transfected HEK293T cells were analyzed by Western blot. THSD7A was detected in both cell lysates and cultured medium. GAPDH served as a cytoplasmic marker. B. Transfected HEK293T cells were incubated in the presence (+) or absence (-) of tunicamycin. After 48 hours, cell lysates and cultured medium were subjected to Western blot and stained with THSD7A-specific and anti-FLAG-tag antibodies (upper panel). The cell lysates and cultured medium of THSD7A-transfected HEH293T cells were treated with (+) or without (-) PNGase and subjected to Western blot (lower panel). C. Cultured medium from transfected HEK293T cells was concentrated by 3 kDa or 100 kDa cutoff Amicon concentrators and analyzed by Western blot. After concentration by a 100 kDa cutoff Amicon concentrator, the 210 kDa soluble THSD7A (arrow) was retained in the concentrates and the 33 kDa amino-terminal fragment of THSD7A (arrowhead) was filtered out. |
|
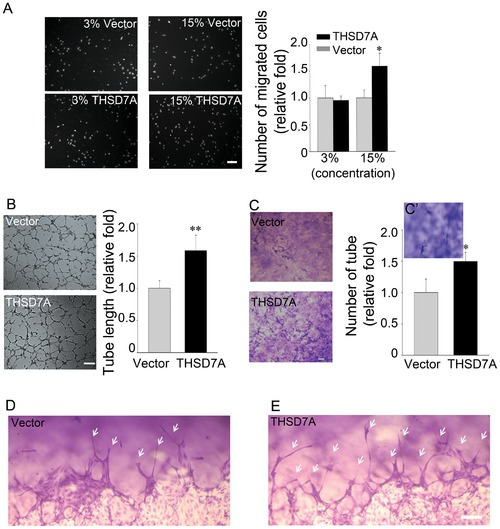
Soluble THSD7A accelerates endothelial cell migration, tube formation and sprouting. A. The motility of HUVEC was evaluated by transwell migration assay in M200 medium. HUVEC were loaded in the upper insert and soluble THSD7A or control medium were added to a 3% or 15% final concentration into the lower chamber. B. Two-dimensional tube formation was performed in the presence or absence of soluble THSD7A on Matrigel. C. Three-dimensional tube formation was performed in the presence or absence of soluble THSD7A on type I collagen as an assay for sprouting. C2. Enlarged image of tubes. Bar represents 10 μm. Each experiment was repeated at least three independent times. *P<0.05 vs. vector. D–E. Side views of the sprouting assay. Arrows indicate tubes. Bar represents 100 μm. |
|
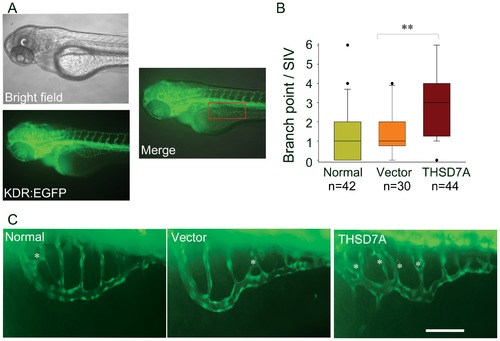
Soluble THSD7A increases the branching of SIVs and leads to an irregular vessel network in zebrafish. A. Images of 74 hpf Tg(kdr:EGFP) zebrafish. The red rectangle indicates the region of the SIV network. B. The median of branch point per SIV network of 74 hpf zebrafish. ** P<0.01 vs. vector. C. Enlarged images of SIV networks. * indicates branch points. Bar represents 100 μm. |
|
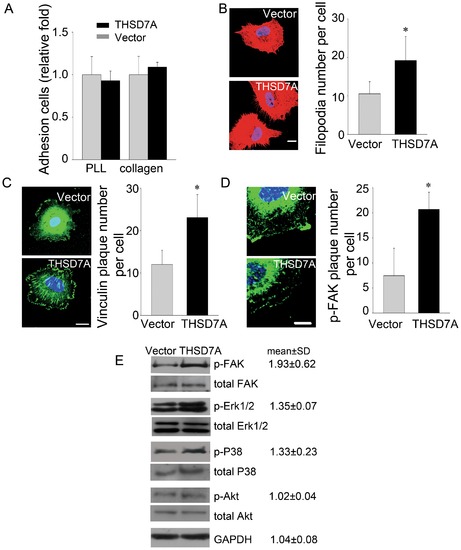
Soluble THSD7A promotes focal adhesion assembly to enhance cell migration via FAK-dependent manner. A. Adhesion assay performed with HUVEC in the presence and absence of soluble THSD7A on type I collagen- or PLL-coated coverslips. B. Immunostaining of actin filaments (red) and nuclei (blue) in HUVEC. The quantitative data show the average number of filopodia per cell. C. Immunostaining of vinculin (green) and nuclei (blue) in HUVEC. The quantitative data show the average number of peripheral vinculin plaques per cell. D. Immunostaining of p-FAK (green) and nuclei (blue) in HUVEC. The quantitative data show the average number of peripheral p-FAK plaques per cell. Bar represents 10 μm. *P<0.05 vs. vector. E, HUVEC lysates treated with soluble THSD7A or control medium were subjected to Western blot. Relative fold levels of p-FAK, p- Erk1/2, p-P38, p-Akt and GAPDH are represented as the mean ± SD. Total levels of FAK, Erk1/2, P38 and Akt served as loading controls. Each experiment was repeated at least three independent times. |
|
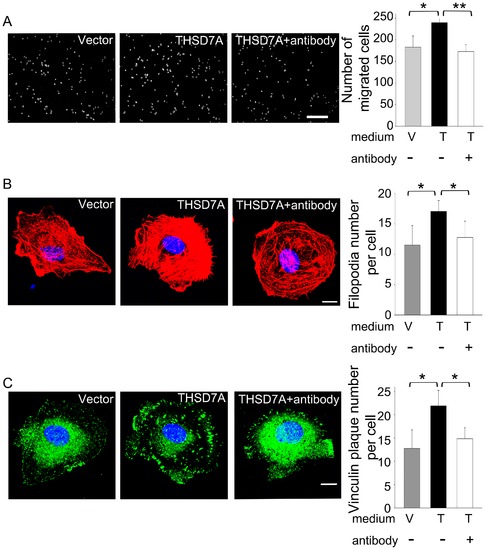
Soluble THSD7A-mediated angiogenic effects are blocked with anti-sTHSD7A antibody. A. The motility of HUVEC was evaluated by transwell migration assay. HUVEC were loaded in the upper insert. Control medium (V) or soluble THSD7A (T) in the presence (+) or absence (-) of anti-sTHSD7A antibody were added to the lower chamber. Bar represents 100 μm. B. Immunostaining of actin filaments (red) and nuclei (blue) in HUVEC treated with control medium (V) or soluble THSD7A (T) in the presence (+) or absence (-) of anti-sTHSD7A antibody. The quantitative data show the average number of filopodia per cell. C. Immunostaining of vinculin (green) and nuclei (blue) in HUVEC treated with control medium (V) or soluble THSD7A (T) in the presence (+) or absence (-) of anti-sTHSD7A antibody. The quantitative data show the average number of peripheral vinculin plaques per cell. Bar represents 10 μm. *P<0.05. **P<0.01. Each experiment was repeated at least three independent times. |
|
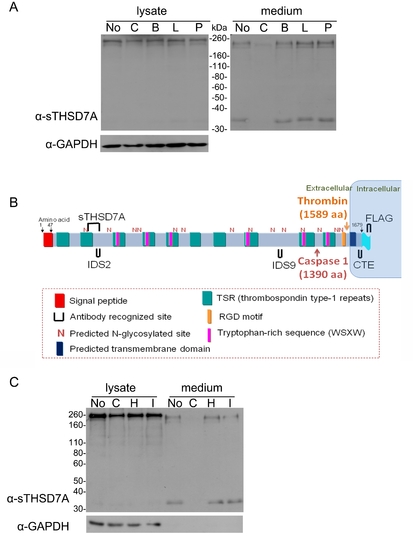
The release of soluble THSD7A is protease-dependent. A. THSD7A-transfected HEK293T cells were treated with complete protease inhibitor (C), Benzamidine (B), Leupeptin (L) and Pepstatin A (P). Untreated cells (No) served as a control. Cell lysates and cultured medium were subjected to Western blot with anti-sTHSD7A antibody. GAPDH served as a loading control. B. Two proteases, thrombin and caspase 1, are predicted to cleave the full-length THSD7A. C. THSD7A-transfected HEK293T cells were treated with complete protease inhibitor (C), hirudin (H), caspase 1 inhibitor I (I) or no treatment (No). Cell lysates and cultured medium were subjected to Western blot with anti-sTHSD7A antibody. GAPDH served as a loading control. |