- Title
-
A highly conserved retinoic acid responsive element controls wt1a expression in the zebrafish pronephros
- Authors
- Bollig, F., Perner, B., Besenbeck, B., Köthe, S., Ebert, C., Taudien, S., and Englert, C.
- Source
- Full text @ Development
|
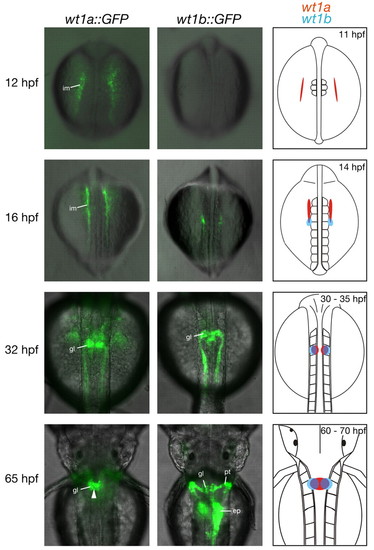
Reporter gene expression in transgenic zebrafish embryos recapitulates expression of wt1 paralogs. Transgenic wt1a::GFP (left) and wt1b::GFP (middle) embryos were imaged using a fluorescence microscope. Shown are overlays of dorsal transmission and fluorescence images at the indicated stages. Arrowhead in the bottom left panel marks fusion of the GFP signal at the midline. The schematic representation of wt1a and wt1b expression on the right is based on published data (Bollig et al., 2006; Drummond et al., 1998; Serluca and Fishman, 2001; Wingert et al., 2007) and is confined to expression in the pronephric region; wt1a expression is shown in red, wt1b expression in blue. ep, exocrine pancreas; gl, glomerulus; hpf, hours post fertilization; im, intermediate mesoderm; pt, pronephric tubule. |
|
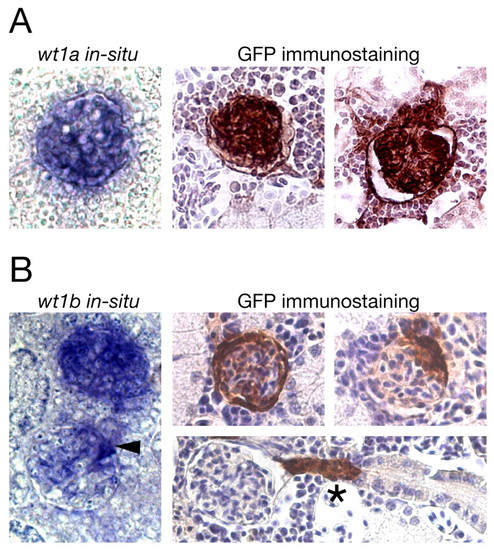
GFP expression in the mesonephros of adult transgenic zebrafish recapitulates expression of wt1 paralogs. (A,B) In situ hybridization for wt1a (A) and wt1b (B) on sections of wild-type mesonephros (left) and GFP immunostainings on sections of wt1a::GFP line 1 (A) and wt1b::GFP line 1 (B) mesonephros (right) are shown. The kidneys were taken from 4- to 6-month old wild-type and transgenic zebrafish. In the immunostainings, cell nuclei are stained blue (Hematoxylin counterstaining) and GFP-positive cells are brown. Arrowhead marks a glomerulus in which only a subset of cells is labeled, asterisk denotes a GFP-positive neck region. |
|
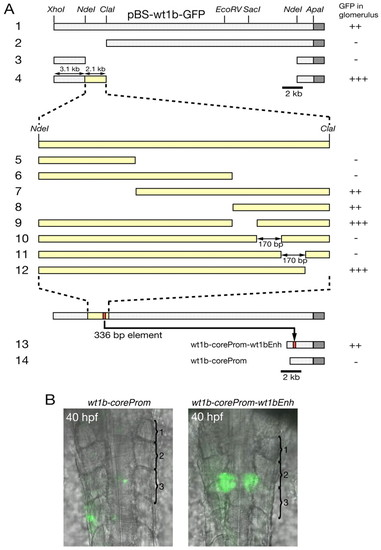
A 336 bp regulatory region upstream of wt1b is necessary and sufficient for expression in the pronephric glomeruli. (A) Deletion constructs were generated using plasmid pBS-wt1b-GFP (row 1). Construct numbers are indicated on the left. Dark gray bars represent the GFP gene, yellow bars represent the 2.1 kb region between NdeI and ClaI sites or subfragments thereof (shown in higher magnification in rows 5-12). At least 40 embryos were investigated for GFP expression in the glomeruli, which are located ventral of the third somite, 24 hours after injection of the respective reporter construct. For quantification, the number of embryos with GFP expression in the glomerulus was divided by the total number of GFP-positive embryos (GFP expression anywhere in the embryo). +++, >40%; ++, 20-40%; -, <5%. (B) Overlay of brightfield transmission and fluorescence images of stable wt1b-coreProm and wt1b-coreProm-wt1bEnh transgenic embryos (dorsal view). The first three somites are numbered and are marked by parentheses. |
|
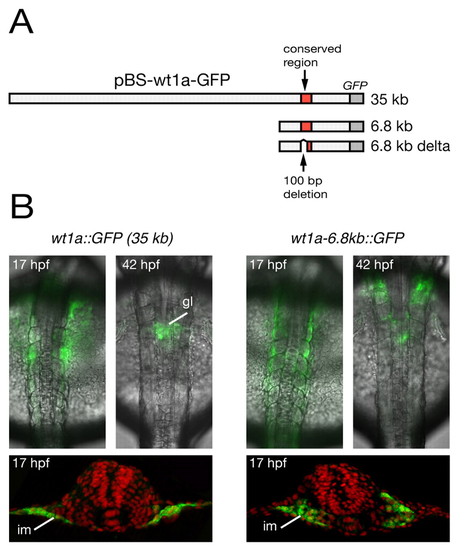
The conserved region upstream of wt1a is required for its expression in the intermediate mesoderm. (A) Schematic representation of wt1a reporter constructs. The upper row represents the plasmid pBS-wt1a-GFP, which was used for the generation of wt1a::GFP transgenic zebrafish. In addition, two truncated wt1a-GFP plasmids are shown containing a 6.8 kb genomic fragment (middle row) and the same fragment lacking 100 bp within the conserved region (lower row). A detailed illustration of the deleted 100 bp region is shown in Fig. 5B. (B) Overlay of brightfield transmission and fluorescence images from wt1a::GFP (left) and wt1a-6.8kb::GFP (right) transgenic embryos at 17 and 42 hpf (top). For detailed analysis, embryos at 17 hpf were stained with anti-GFP antibody (green) and were sectioned (bottom). Counterstaining with DAPI is shown in false color (red). gl, glomeruli; im, intermediate mesoderm. |
|
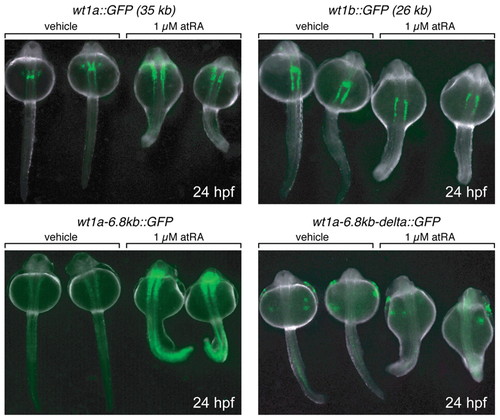
Retinoic acid acts via the conserved region upstream of wt1a. Embryos of the indicated transgenic lines were treated either with 1 μM all-trans retinoic acid (atRA) or 0.1% DMSO (vehicle) from 12 hpf (6-somite stage) to 24 hpf. Subsequently, embryos were embedded in 3% methyl cellulose and imaged with a fluorescence stereomicroscope (dorsal view). RA- and vehicle-treated embryos of each line were captured together. Note that the weak fluorescence signal of the vehicle-treated wt1a-6.8kb::GFP embryos is due to the short exposure time used for recording the image. |
|
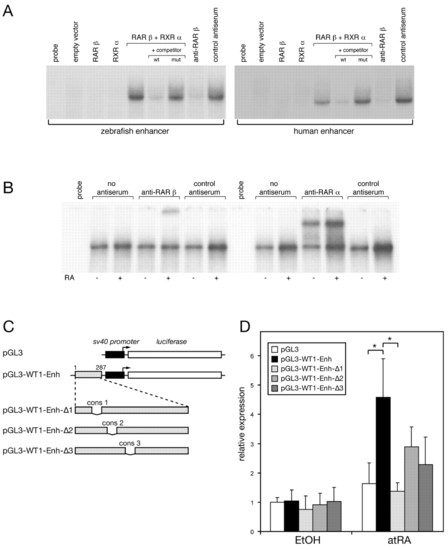
Characterization of the retinoic-acid-responsive Wt1 enhancer. (A) Electrophoretic mobility shift assay (EMSA) using in vitro transcription/translation (TNT) products of RARβ and RXRα on the zebrafish (left) and the human (right) enhancer. TNT extract programmed with the empty Rc/CMV vector was used as a negative control. Mutant fragments for competition experiments harbored a deletion of conserved region 1 (cons 1) in the background of the zebrafish or human enhancer. For supershifts, an antibody against RARβ or control antiserum was used. (B) Supershift assays using the human WT1 enhancer fragment as the probe. Nuclear extracts were prepared from P19 cells treated with RA (+) or ethanol (-) for 24 hours. Analysis was performed using antibodies specific for RARβ and RARα or respective control antibodies. The different mobility of the RARβ and RARα supershift complexes is most likely to be explained by the antibodies used (monoclonal versus polyclonal antiserum). Note that pre-incubation of the extracts with RARβ antiserum resulted in either immunodepletion (A) or supershift (B) of the DNA-protein complexes, depending on the type of extract. (C) Schematic luciferase reporter constructs. The sequence of the inserted human WT1 enhancer region (287 bp) and of the deleted conserved elements (cons 1-3) is shown in Fig. 5B. (D) P19 cells were transfected with the constructs shown in A and grown for 4 days in the presence of 1 μM all-trans retinoic acid (atRA) or vehicle (0.1% ethanol). Subsequently, cells were lysed and luciferase activity was measured. Luciferase expression in vehicle-treated cells transfected with pGL3 vector was set to one. The figure shows one representative experiment. Data represent the means and standard deviations of triplicates. Asterisks indicate statistically significant differences, *P<0.05, Student′s t test. mut, mutant; wt, wild type. |
|
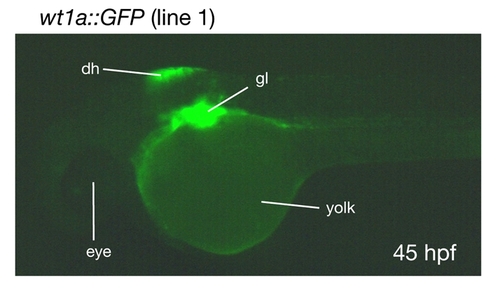
wt1a::GFP embryos show GFP expression in the dorsal hindbrain. A lateral image of a wt1a::GFP line 1 embryo was recorded 45 hours post fertilization (hpf). dh, dorsal hindbrain; gl, glomerulus. |
|
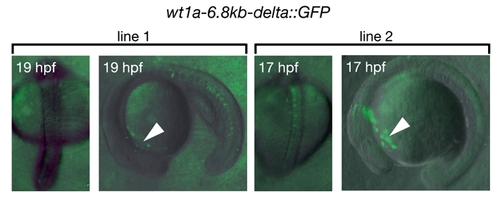
Embryos of two stable transgenic lines harboring the wt1a-6.8kb-delta::GFP construct. These lines could be isolated only by the very weak GFP expression in cells located at the anterior yolk region (arrowheads) and did not show expression in the intermediate mesoderm. |