- Title
-
Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development
- Authors
- Skouloudaki, K., Puetz, M., Simons, M., Courbard, J.R., Boehlke, C., Hartleben, B., Engel, C., Moeller, M.J., Englert, C., Bollig, F., Schäfer, T., Ramachandran, H., Mlodzik, M., Huber, T.B., Kuehn, E.W., Kim, E., Kramer-Zucker, A., and Walz, G.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
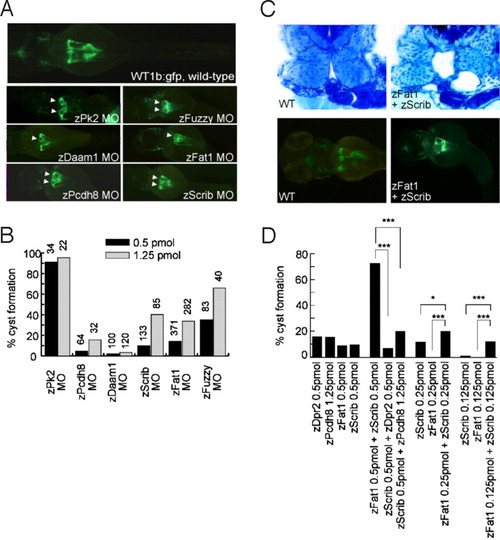
Zebrafish Fat1 genetically interacts with zebrafish Scribble. (A) MO-induced knockdown of zFat1 was compared with the knockdown of zebrafish Prickle 2 (zPk2), zebrafish Daam1 (zDaam1), zebrafish Protocadherin 8 (zPcdh8), zebrafish Fuzzy (zfuzzy), and zScrib. White arrowheads indicate examples of cyst formation. (B) Pronephric cyst formation, caused by the depletion of these molecules, was scored at 55 h post fertilization (h.p.f), using the transgenic zebrafish line Wt1b:gfp. A reproducible degree of cyst formation (30%–40%) was noted in zFat1-depleted embryos without significant reduction in larval survival. (C) Epistasis assays between components of the PCP pathway revealed a strong synergism between zFat1 MO (0.125 pmol) and zScrib MO (0.125 pmol) injections. Transverse sections at the level of glomerulus and proximal tubules revealed bilateral pronephric cyst formation adjacent to the glomerulus in combined knockdown of Fat1 and Scribble in zebrafish embryos. (D) The presence of pronephric cysts was scored at 50–55 h.p.f. in zebrafish embryos injected with low MO concentrations. Note that pronephric cysts are hardly detectable after single injections of either 0.125 pmol zFat1 MO or 0.125 pmol zScrib MO, whereas the combination causes pronephric cysts in > 10% of microinjected embryos. (*, P < 0.05; **, P < 0.0001). PHENOTYPE:
|
|
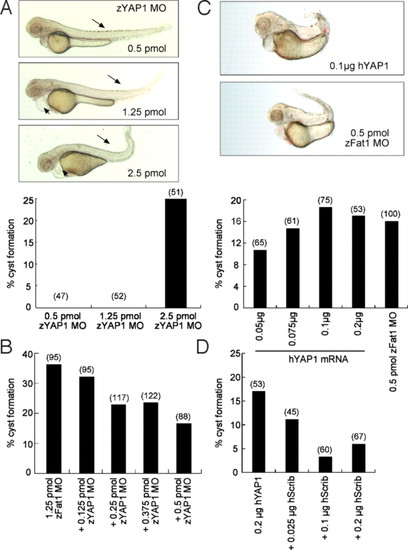
Control of YAP1 expression is essential for normal zebrafish pronephros development. (A) Knockdown of zYAP1 by MO (zYAP1 MO) results in pronephric cysts. Lateral view of embryos injected with zYAP1 MO (0.5 pmol, 1.25 pmol, and 2.5 pmol) at 55 h.p.f. A curled tail and shortening of the body (arrows) are observed in the zYAP1 morphants (Upper). At higher concentration, zYAP1 MO injections led to cyst formation (Lower). (B) Cyst formation caused by zFat1 MO was partially rescued by the simultaneous knockdown of zYAP1. (C) Overexpression of human YAP1 (hYAP1) recapitulated the loss-of-function phenotypes (cyst formation) caused by zFat1 knockdown (zFat1 MO). (D) Over-expression of human YAP1 resulted in pronephric cysts in zebrafish embryos. This result was reversed by co-injection of human Scribble RNA (hScrib). (Numbers in parentheses indicate the total number of embryos used in each experiment.) PHENOTYPE:
|
|
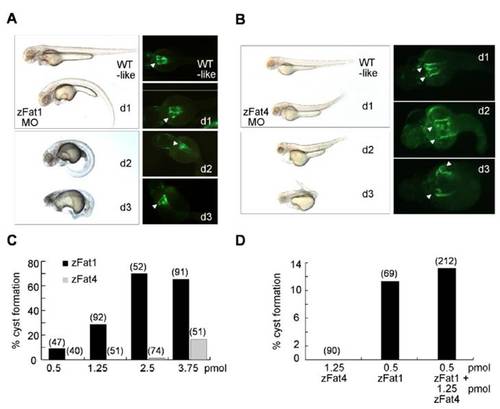
Knockdown of zFat1 and zFat4 promotes cyst formation within the zebrafish pronephros. (A, B) Depletion of zFat1 and zFat4 by MO directed against the translational start site results in pronephric cyst formation and abnormal body curvature. The injected larvae were classified into 3 different groups according to the degree of their dysmorphic changes (d1–d3). Pronephric cysts (Arrowheads) were visualized in the proximal tubules, using a Wt1b::GFP transgenic zebrafish line. (C) The comparison between zFat1 (zFat1 MO) and zFat4 (zFat4 MO) reveals that knockdown of zFat1 causes a higher rate of pronephric cyst formation than zFat4 depletion. (D) The combined knockdown of zFat1 and zFat4, using 0.5 pmol and 1.25 pmol of MO, respectively, did not reveal a significant synergistic effect between both MO when compared with a control MO.(Numbers in parentheses indicate the total number of embryos used in each experiment.) |
|
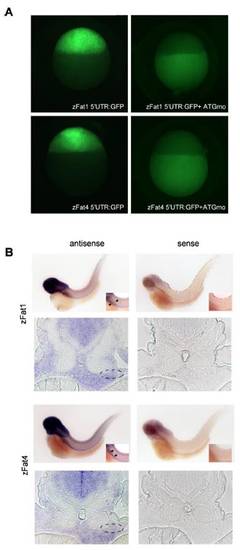
Efficacy of zFat1 and zFat4MO(A) and the expression pattern of zFat1 and zFat4 (B). (A) The green fluorescence, caused by injection of 5′UTR-Fat1:GFP and 5′UTR-Fat4:GFP, was equally abolished by co-injection of zFat1 MOand zFat4 MO. The analysis was performed 4 h.p.f. at sphere stage. (B) Expression of zFat1 and zFat4 detected by in situ hybridization. Digoxigenin-labeled anti-sense probes against the EGF repeat (12562–13056 bp) of zFat1 and the C-terminus of zFat4 were used 55 h.p.f. Both zFat1 and zFat4 transcripts were detectable in the developing pronephros (indicated by arrows and circles). The specificity of each probe was confirmed by in situ hybridization with the corresponding zFat1 and zFat4 sense probe. EXPRESSION / LABELING:
|
|
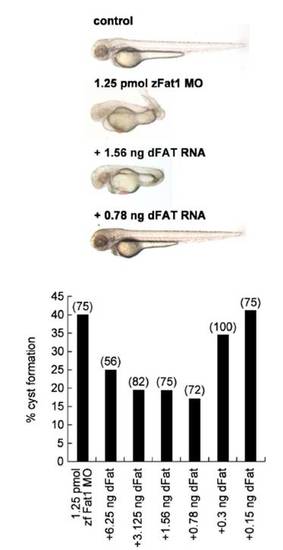
A Drosophila Fat truncation (sIg.TM.dFat) rescues the phenotypic changes caused by zFat1 depletion in zebrafish. (Upper) Morphological alterations of zFAT1-MO injected embryos included curled tails and edema. Co-injection with Drosophila Fat RNA rescued the observed phenotypic defects (embryos shown correspond to different amounts of injected RNA in lower panel). (Lower) Titration of the amount of co-injected Drosophila Fat RNA revealed a statistically significant reversal of the cystic phenotype in at least half of the analyzed embryos. (Numbers in parentheses indicate the total number of embryos used in each experiment.) |
|
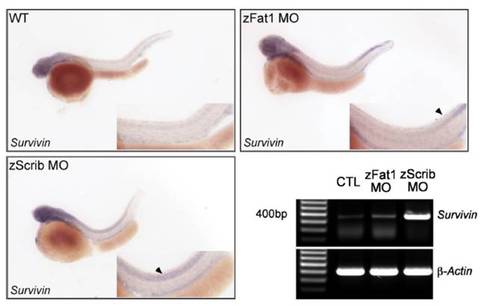
Whole-mount in situ hybridization and RT-PCR were carried out for wild-type and MO-injected (zFat1MOand zScrib MO) zebrafish embryos. Embryos were hybridized with Survivin anti-sense mRNA at 48 h.p.f using identical conditions and exposure times. Arrowheads indicate regions of the zebrafish embryo with increased levels of Survivin expression in the zScrib MO- and zFat1 MO-injected embryos. The up-regulation of Survivin in zScrib MO- and zFat1 morphants was confirmed by RT-PCR at 48 h.p.f. |
|
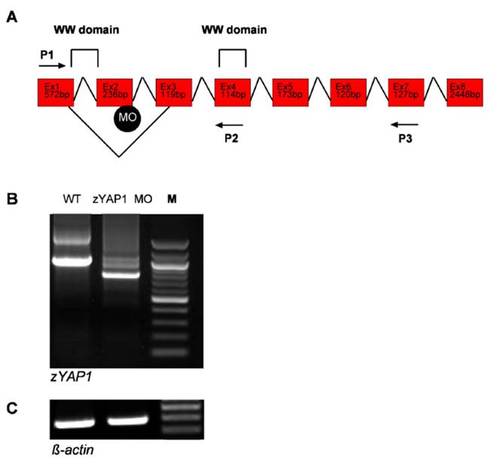
Defective splicing within theWWdomain of zebrafish zYAP1. (A) Schematic representation of zYAP1 and the MO-induced splicing defect within the first WW domain. The WW domain is encoded by Exon1 (Ex1) and Exon2 (Ex2). Position of the MO targeted against the donor splice site is indicated. Two sets of PCR primer combinations, P1/P3 and P1/P2, were used to detect the splice defect. (B) Analysis of the splicing defect induced by the zYAP1 MO. RT-PCR of YAP1 at 55 h.p.f generated a 1031-bp fragment in wild-type embryos (Lane WT), and a 777-bp fragment in embryos injected with zYAP1 MO (Lane zYAP1 MO). M, 100-bp marker. The defective splicing resulted in preterminal stop of the protein. (C) RT-PCR of β-actin mRNA was performed to control for equal amounts of RNA in each sample. |