- Title
-
The embryonic expression patterns and the knockdown phenotypes of zebrafish ADP-ribosylation factor-like 6 interacting protein gene
- Authors
- Huang, H.Y., Dai, E.S., Liu, J.T., Tu, C.T., Yang, T.C., and Tsai, H.J.
- Source
- Full text @ Dev. Dyn.
|
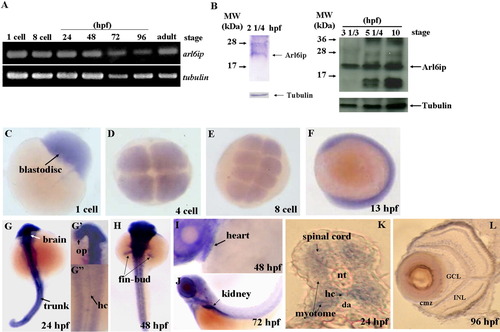
Detection of the existence of arl6ip transcript and protein in zebrafish embryos using RT-PCR (A) and Western blot (B), respectively. The spatial expression of arl6ip mRNA was detected by whole-mount in situ hybridization (C-L). The arl6ip mRNA was first detected in blastodisc at the 1-cell stage (A,C), suggesting that the arl6ip transcript is maternally inherited. RT-PCR revealed that the arl6ip transcript was also detected at the 8-cell stage, 24, 48, 72, 96 hpf, and adulthood, when tubulin mRNA was used as a positive control (A). Total proteins were extracted from the wild-type embryos at 22 1/2, 3 1/3, 5 1/4 or 10 hpf, and the rabbit polyclonal antibody against ARMER was used to perform Western blot analysis when endogenous β-Tubulin was used as a control (B). Arl6ip was present at 2 1/4, 3 1/3, 5 1/4, and 10 hpf (indicated by arrow). Whole-mount in situ hybridization showed that the arl6ip was expressed ubiquitously in developing embryos at the 1-, 4-, and 8-cell stages and 13 hpf (C-F). The arl6ip was displayed in the brain, optic primordia (op), spinal cord, myotome, hypochord (hc), trunk, fin-bud, heart, kidney, and retina from 24 to 96 hpf (G-L). Dorsal view of arl6ip staining (G-G″,H). Lateral view of arl6ip expressions (F,I,J). arl6ip was expressed in retina by cross-sectioning of the eyes at 96 hpf (L). nt: notochord; da: dorsal aorta; GCL: ganglion cell layer; INL: inner nuclear layer; cmz: ciliary marginal zone; L: lens; hpf: hours post-fertilization of zebrafish embryos. |
|
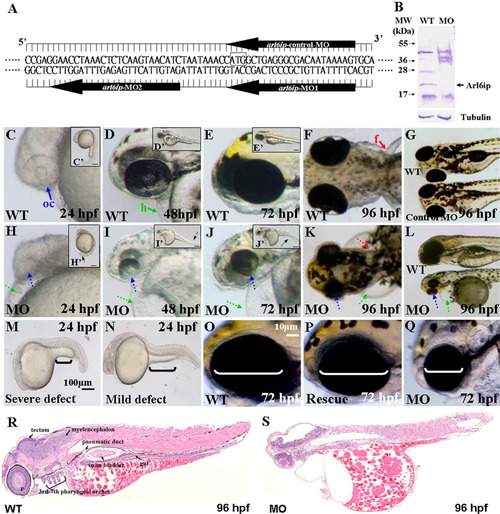
A: Nucleotide sequences of the designed arl6ip-morpholino (MO). The arl6ip-MO1 targets the coding region of arl6ip mRNA, and arl6ip-MO2 targets the 5′-untranslated region. The arl6ip-control-MO served as a negative control MO, which was an inverted MO sequence of arl6ip-MO1. Square box, Arl6ip translation start site. B: Western blot analysis of total proteins extracted from the wild-type (WT) and the arl6ip-MO1-injected (MO) embryos, with β-Tubulin used as a control. The endogenous Arl6ip (arrow) from the arl6ip-morphants was completely absent. C-N: Phenotypic defects in the arl6ip-MO1-injected embryos were observed at the stages indicated. They were microphthalmia (small eyes; dotted blue arrow), pericardial edema (dotted green arrow), flat head, deformed trunk (dotted black arrow in H′-J′), pigment pattern disorder and pectoral fin defect (dotted red arrow). Black brackets indicate the extended yolk length by which we identified the different defect grades (M,N). Scale bar = 100 μm in M,N. oc, optic cup (solid blue arrow); h, heart (solid green arrow); f, fin (solid red arrow) were indicated in wild-type embryos. Scale bar = 10 μm in C′-E′,H′-J′,O-Q. The defective arl6ip-morphants (Q) were partially rescued to develop the wild-type-like phenotype (P) after co-injecting the synthetic arl6ip-mRNA with arl6ip-MO2. The white brackets indicate the eye dimensions. The histological sections were examined for the wild-type embryos and the arl6ip-morphants at 96 hpf after hematoxylin-eosin staining (R,S). Compared to the tissue morphology of wild-type embryos, arl6ip-morphants displayed many abnormalities of tissue development, including retina, tectum, myelencephalon, 3rd-7th pharyngeal arches, pneumatic duct, swim bladder, and gut. EXPRESSION / LABELING:
|
|
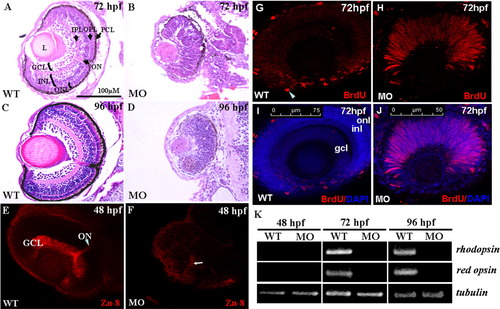
A-D: The histological examination of zebrafish retinae. Histological section and hematoxylin-eosin staining of retinae were performed on wild-type embryos and arl6ip-morphants at 72 and 96 hpf. Unlike the wild-type embryos (A,C), the neuronal differentiation is severely delayed, resulting in no formation of retinal layers in the arl6ip-morphants, both at 72 (B) and 96 hpf (D). In addition, retina development of arl6ip-morphants was markedly retarded. GCL, ganglion cell layer; INL, inner nuclear layer; L, lens; MO, arl6ip-morphant; ONL, outer nuclear layer; ON, optic nerve; OPL, outer plexiform layer; IPL, inner plexiform layer; PCL, pigment cell layer; WT, wild-type. The scale bar = 100 μm. E,F: Knockdown of Arl6ip causes the defective formation of the retinal axons in zebrafish embryos. By using the confocal fluorescence microscope, we observed that Zn8 protein was detected in the retinal ganglion cell axons of wild-type (WT) embryos at 48 hpf (E). However, the protein level of Zn8 presented a very weak signal near the retinal ventral (F, arrow) in the embryos injected with 4 ng/embryo of arl6ip-MO1 (MO). GCL, ganglion cell layer; ON, optic nerve. G-J: The retinal cells stayed at S-phase in retinal developmental period in zebrafish embryos when Arl6ip was lost. BrdU (red) and DAPI (blue) were used to label the S-phase of cell cycle and the nuclear, respectively. There was no BrdU signal detected in the outer nuclear layer (onl), inner nuclear layer (inl), and ganglion cell layer (gcl) (G,I) of wild-type embryos (WT) at 72 hpf, except the ciliary marginal zone (cmz, arrowhead). However, at the same stage, in embryos injected with 4 ng/embryo of arl6ip-MO (MO), we found that there was a large number of BrdU signal in retina (H,J), indicating that the retina cells of arl6ip-morphant were maintained at S-phase. The images were taken by confocal microscopy. The scale bars = 75 μm (G,I) and 50 μm (H,J). K: Detection of the transcripts of rhodopsin and red opsin mRNA of zebrafish embryos using RT-PCR. After the total RNA was extracted from wild-type embryos and arl6ip-morphants at 48, 72, and 96 hpf, RT-PCR was used to detect the existence of the transcripts of rhodopsin, which are expressed exclusively in rods and red cones, respectively. The expression of tubulin served as positive control. Results showed that neither rhodopsin nor red opsin transcripts were detected in either the wild-type embryos or arl6ip-morphants at 48 hpf as a result of the fact that the opsin gene is not transcribed until 50 hpf in the retina. After starting transcription of rhodopsin mRNA and red opsin mRNA at, for example, 72 and 96 hpf, the transcripts of rhodopsin and red opsin were present in the wild-type embryos. However, both rhodopsin and red opsin mRNA were totally absent in the arl6ip-morphants. WT, wild-type; MO, arl6ip-morphants. |
|
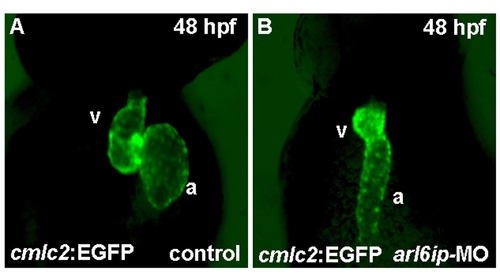
Zebrafish transgenic line Tg(cmlc2::EGFP) (Huang et al., 2003) was used to observe heart development. When we injected arl6ip-MO1 (MO) into embryos derived from this line and observed at 48 hpf by confocal microscopy, we found that the heart looping was defective. a, atrium; v, ventricle. |
|
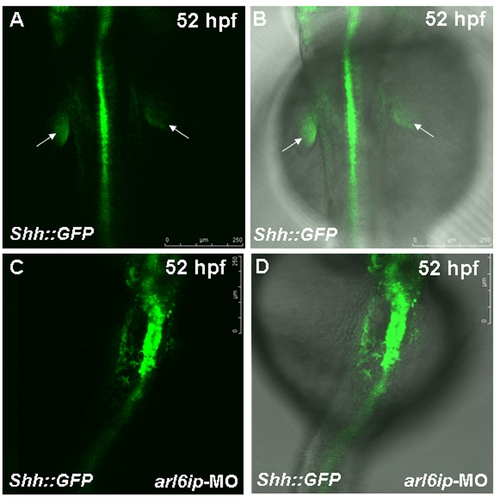
Zebrafish transgenic line Tg(shh::EGFP) (Shkumatava et al., 2004) was used to observe the fin-bud development. The white arrow indicates the fin-bud of wild-type (WT) (A,B). When we injected arl6ip-MO1 (MO) into embryos derived from this line and observed at 52 hpf by confocal microscopy, we found that the fin-bud development was defective (C,D). Embryos were photographed under blue light (A,C) and bright field overlapped with blue light (B,D). The scale bar = 250 μm. |

Unillustrated author statements |