- Title
-
Histamine metabolism influences blood vessel branching in zebrafish reg6 mutants
- Authors
- Huang, C.C., Huang, C.W., Cheng, Y.S., and Yu, J.
- Source
- Full text @ BMC Dev. Biol.
|
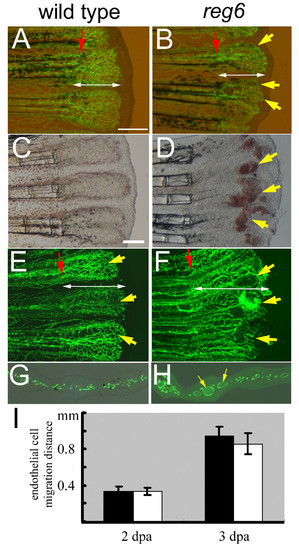
Normal migration of regenerating endothelial cells in zebrafish reg6 mutants. (A, B) Combined bright field and green fluorescent images of wild-type TG(fli1:EGFP)y1 (A) and reg6;TG(fli1:EGFP)y1 (B) regenerating fins at 2 days post amputation (dpa) showing the distance (designated by white double arrows) that the blood vessel endothelial cells (ECs) have migrated. Yellow arrows, dilated vessels of reg6 mutants. (C-F) In 3-dpa regenerates, wild-type fish have formed normal capillary plexuses (C, yellow arrows in E)., but reg6 fish (D, bright field; F, green fluorescent) have developed numerous swollen vessels (yellow arrows in D and F) resulting in blood cell accumulation (red spots in D). (G, H) Cross-sections of 3-dpa regenerates showing normal regenerating vessels in wild-type (G) and dilated vessels in reg6 fish (H, yellow arrows). Red arrows, amputation plane. Scale bars, 300 μm. (I) Quantitative data of the migration distance of regenerating ECs in wild-type (black bars) and reg6 fish (white bars) at 2 and 3 dpa. |
|
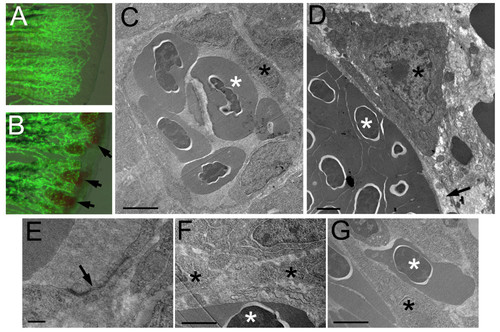
Cellular defects of reg6 endothelial cells. (A, B) Overlaid images of bright field and green fluorescent protein (GFP) of wild-type (A) and reg6 (B) 3-dpa regenerating fins showing the leakage of blood cells in reg6 fish (arrows). Transmission electron microscopic images of wild-type fish (C, low magnification; E, high magnification) showing that the blood vessel is enclosed by several endothelial cells which form nice, intact tight junctions (arrow in E). Additional images can be found in Additional file 1. In contrast, the blood vessels in reg6 fish (D) were usually leaking as evidenced by the lack of coverage by endothelial cells (arrow) and the presence of blood cells outside blood vessels (as an endothelial cell is sandwiched by two blood cells in G and arrows in Additional file 2). In addition, reg6 endothelial cells appeared less stretched-out (D), and tight junction were rarely formed among them (F). Scale bars, 2 μm for C, D, F, and G; 10 nm for E. white asterisks, blood cell; black asterisks, endothelial cell. PHENOTYPE:
|
|
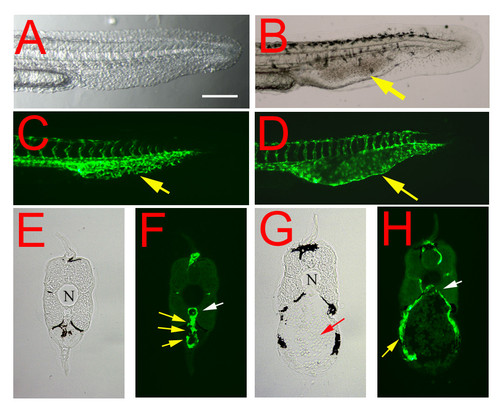
Embryonic phenotype of the reg6 mutation. (A, C) In a 36-hpf (hours post fertilization) wild-type embryo (A, bright field; C, green fluorescent), the developing caudal veins form vascular plexuses (yellow arrow in C). (B, D) In contrast, endothelial cells of reg6 embryos (B, bright field; D, green fluorescent) develop fewer vessel branches and form swollen lumina (yellow arrows). Lateral view; anterior, left; dorsal, top. Scale bar, 100 μm for A-D. (E-H) Cross-sections of the tail regions showing multiple lumina of the capillary plexus of a wild-type caudal vein (E, yellow arrows in F) but a single enlarged lumen in reg6 (G, yellow arrow in H). N, notochord; white arrows, dorsal aorta; yellow arrows, caudal vein; red arrow, accumulated blood cells. |
|
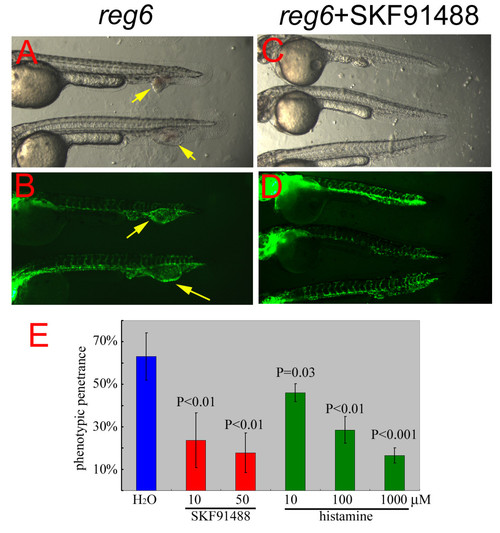
Suppression of the reg6 embryonic phenotype by SKF91488 and histamine. (A, bright field; B, GFP) Untreated reg6 embryos developed dilated caudal veins at 33°C (yellow arrows). (C, bright field; D, GFP) reg6 embryos treated with 10 μM HMT inhibitor SKF91488 developed normal caudal veins. Anterior, left; dorsal, top. (E) Typically, almost 70% of reg6 embryos formed dilated caudal veins at 33°C (blue bar). The penetrance was reduced by SKF91488 (red bars, n = 50) and histamine (green bars, n = 50). |
|
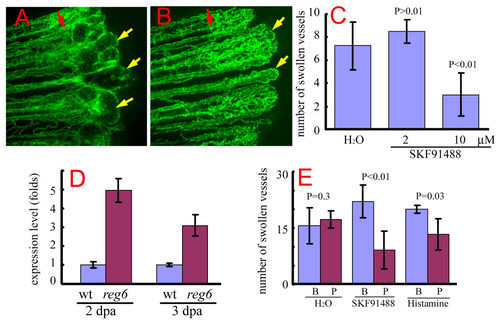
Suppression of the reg6 regenerating vessel phenotype by SKF91488 and histamine. (A) reg6 regenerating vessels formed swollen lumina 3 days post amputation (dpa) at 33°C (yellow arrows). (B) In the presence of 10 μM SKF91488, reg6 mutants developed significantly smaller and fewer swollen vessels (yellow arrows). Red arrows, amputation plane. (C) Quantitative data of the suppression of reg6 regenerating vessels by SKF91488 (n = 5 for each group). This experiment was repeated at least 3 times, and consistent results were observed. (D) Quantitative polymerase chain reaction (QPCR) analyses showed elevated levels of histamine methyltransferase (HMT) RNA in both reg6 2 and 3-dpa regenerates compared with the wild-type. (E) Both SKF91488 and histamine could reverse the reg6 vessel phenotypes. The number of swollen vessels in reg6 mutants was counted at 3 dpa at 33°C (blue bars; B, before treatment) before they were transferred to either fresh water (H2O), 10 μM SKF91488, or 1 mM histamine. The number of swollen vessels was counted again 12 h later (red bars; P, post-treatment). Shown is a representative set of data with 10 fish. Similar results were obtained from 2 independent experiments with different stocks of reg6 fish. |
|
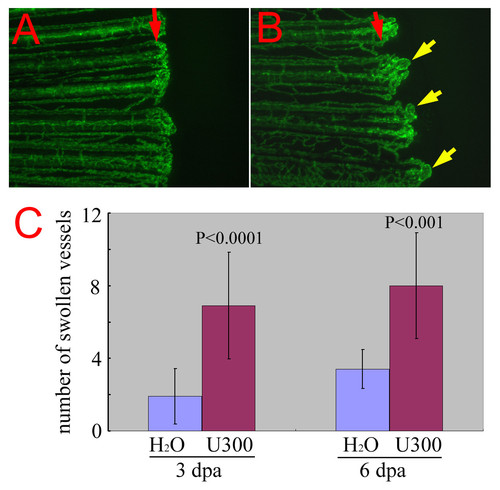
Chemical enhancer of reg6 mutation. (A) reg6 is a temperature-sensitive mutation and regenerates almost normally at 20°C. Shown is the regenerating vessels 6 days post amputation at 20°C. Note that they regenerated much more slowly at the low temperature. (B) When treated with 300 μM of the histidine decarboxylase inhibitor, urocanic acid, reg6 siblings developed a significant number of swollen vessels (yellow arrows). Red arrows, amputation plane. (C) The enhancement by urocanic acid of reg6 vessel defects was evaluated at two regenerative stages, during anastomosis (3 dpa at 20°C, n = 10) and plexus formation (6 dpa at 20°C, n = 10) that are both affected by the reg6 mutation [16]. U300, 300 μM of urocanic acid. |
|
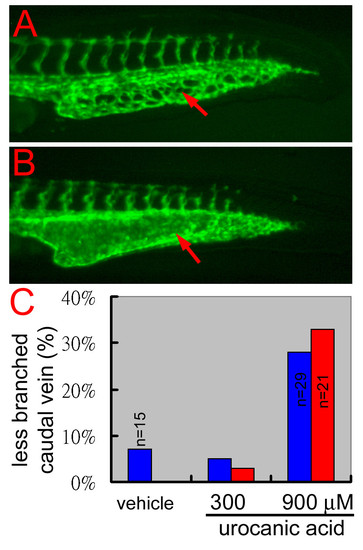
Blocking of histamine synthesis causes blood vessel branching defects in wild-type zebrafish embryos. (A) In a 32 hpf wild-type embryo, the developing caudal vein formed a transient vascular plexus. (B) When treated with 900 μM of the histamine synthesis blocker, urocanic acid, the caudal vein of a wild-type sibling formed much fewer vascular branches and even developed a reg6-like swollen lumen. Red arrows, caudal veins. (C) Quantitative results of the suppression of urocanic acid on blood vessel branching in wild-type zebrafish embryos. Two clutches of wild-type embryos (blue and red bars) were treated with the vehicle (0.0015 hydrochloric acid), or 300 or 900 μM of urocanic acid in egg water, from 26–36 hpf at 28.5°C. The percentage of embryos with less branched caudal vein was scored at the end of treatment (Y-axis, n = 40 unless otherwise specified). |
|
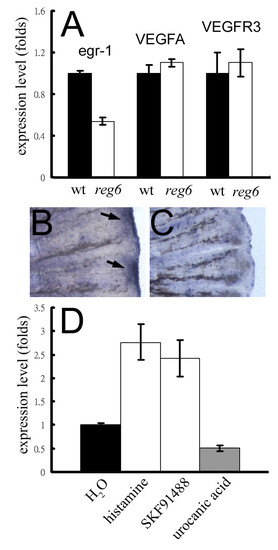
Association of egr-1 expression and the reg6 vessel branching defect. (A) Expression levels of the egr-1 gene in wild-type (wt; black bars) and reg6 (white bars) 3-dpa regenerates. (B, C) Whole-mount in situ hybridization for egr-1 on wild-type (B) and reg6 (C) 3-dpa regenerates showed decreased expression of egr-1 in reg6 fish. Arrows, egr-1 RNA signal. (D) The egr-1 expression in reg6 mutants was upregulated more than 2.5-fold by 1 mM histamine and 10 μM SKF91488 but was downregulated by 300 μM urocanic acid. The expression level was determined by QPCR and normalized by the expression level of the β-actin gene. EXPRESSION / LABELING:
|
|
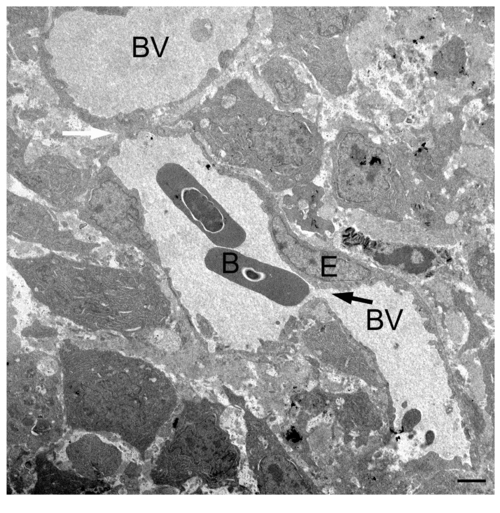
TEM image of the regenerating blood vessels of wild-type fish. A representative TEM image of a wild-type 3-dpa regenerate shows vascular plexus evident by the narrow canal (black arrow) and connection (white arrow) between blood vessels that are of different sizes. B, blood cell; E, endothelial cell; BV, blood vessel. Scale bar, 2 μm. |
|
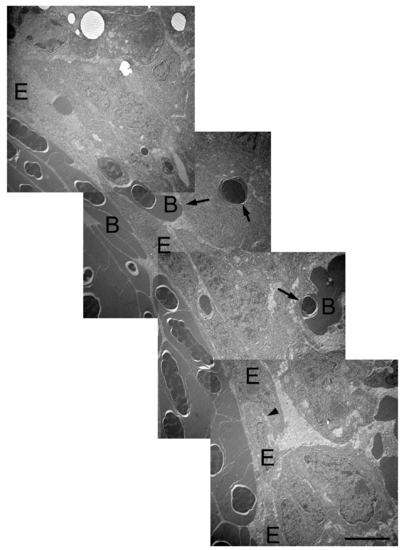
TEM images of the regenerating blood vessels of reg6 fish. Composite TEM images of a reg6 3-dpa regenerate show enlarged blood vessel (lower left cornor) which is filled with blood cells. Blood cells are often leaked out of the blood vessels (arrows). The endothelial cells in reg6 are less stretch-out and sometimes stacked (arrowhead). However, no clear cell junction is found among these cells. B, blood cell; E, endothelial cell. Scale bar, 2 μm. PHENOTYPE:
|
|
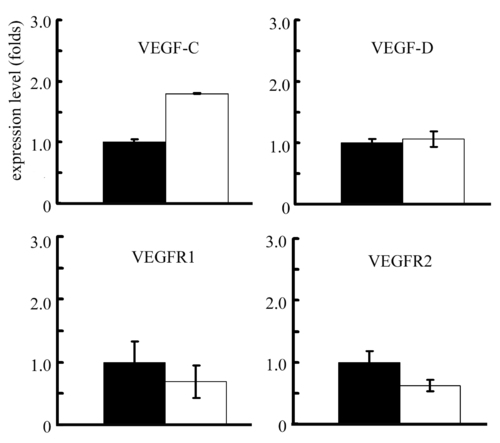
Expression levels of VEGF related genes in wild-type and reg6 regenerative fins. The expression levels of VEGFC, VEGFD, VEGFR1, and VEGFR2 is about the same in wild-type (black bars) and reg6 (white bars) 3-dpa regenerates. Note that the level of VEGFC seems higher but the magnitude is less than two folds. The expression level is determined by QPCR and normalized by the expression level of β-actin gene. EXPRESSION / LABELING:
|
|
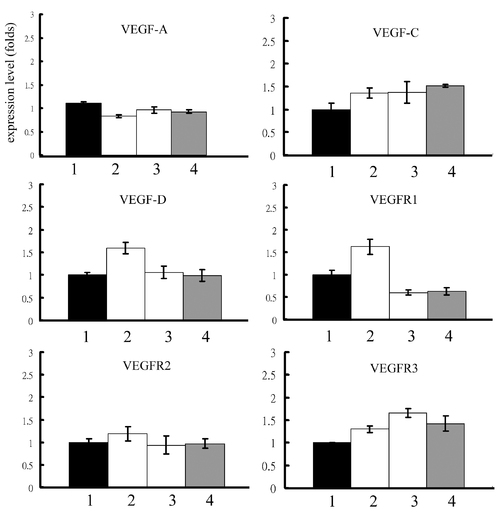
Expression levels of VEGF related genes in reg6 regenerative fins upon different treatments. The treatment of (1) H2O, (2) 1 mM histamine, (3) 10 μM SKF91488, or (4) 300 μM urocanic acid does not alter significantly the expression levels of VEGF-A, VEGF-C, VEGF-D, VEGFR1, VEGFR2, and VEGFR3 in reg6 3-dpa regenerates. EXPRESSION / LABELING:
|