- Title
-
The role of the SPT6 chromatin remodeling factor in zebrafish embryogenesis
- Authors
- Kok, F.O., Oster, E., Mentzer, L., Hsieh, J.C., Henry, C.A., and Sirotkin, H.I.
- Source
- Full text @ Dev. Biol.
|
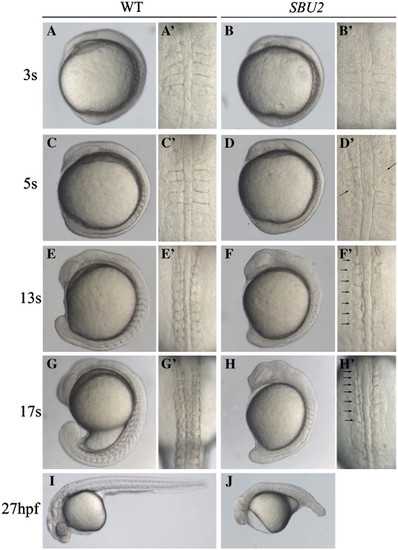
Phenotype of SBU2 mutants. (A–J) Lateral views of living WT and SBU2 embryos, anterior to the top and dorsal to the right. (A′–H′) Dorsal views of panels A–H, anterior to the top. At early somitogenesis, somite boundaries are irregular and indefinite (A′ and B′) in SBU2 embryos. At ∼ 5-somite stage, somites are formed asymmetrically while previously formed somite boundaries start to degenerate (arrows) (C′ and D′). By the 5-somite stage SBU2 mutants have a slightly shorter body length then their wild-type siblings (C and D) and by the 13-somite stage, SBU2 mutants are clearly less extended along A–P axis than wild-type embryos (E and F). While somites continue to form in wild-type embryos, SBU2 mutants have only irregularly formed 6–7 somites and lack posterior somites (arrows) (F′ and H′). By 24–27 hpf, most of the previously formed structures including somite boundaries are degraded or distorted and the embryos degenerate (J). Note: all of the embryos shown in this figure and the following figures are diploids. PHENOTYPE:
|
|
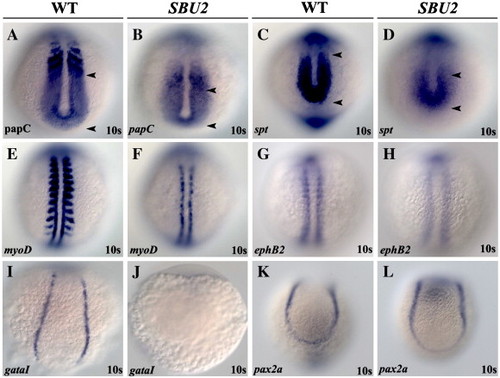
SBU2 mutants have mesoderm defects. Whole mount RNA in situ hybridization of mesodermal markers in wild-type and SBU2 mutants. All views are dorsal; anterior to the top. The expression domains of presomitic mesoderm markers papC and spt are reduced in SBU2 mutants (A–D). A–P patterning of the somites in SBU2 embryos is also affected; myoD expression at the posterior half of the somites is lost while it is reduced at adaxial cells (E and F), ephB2 at the anterior half of the somites is reduced and more diffused (G and H). gataI expression is diminished in SBU2 mutants (I and J) indicating that lateral plate mesoderm development is highly reduced. There is no apparent difference in pax2a expression between wild-type and SBU2 mutants (K and L); SBU2 mutants have no defect in intermediate mesoderm formation. The most anterior and posterior papC and spt expression is marked by arrowheads. EXPRESSION / LABELING:
|
|
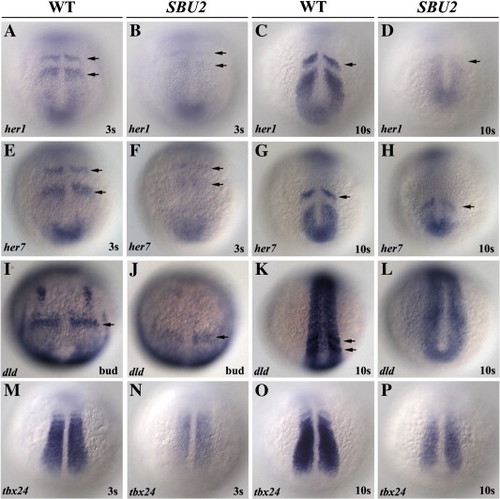
Somite segmentation and PSM maturation are disrupted in SBU2 mutants. Whole mount RNA in situ hybridization of genes involved in somite segmentation process in wild-type and SBU2 mutants. All views are dorsal; anterior to the top. In wild-type embryos, periodic activation of notch signaling provides cycling gene expression of Notch pathway genes such as her1 and her7 (A–D and E–H). dld expression is weak and diffused in presomitic mesoderm as well as anteriormost somites (I–L). tbx24 normally expressed in intermediate and anterior PSM, is also reduced in SBU2embryos (M–P). Arrows denote stripes of her1, her7 or dld expression. EXPRESSION / LABELING:
|
|
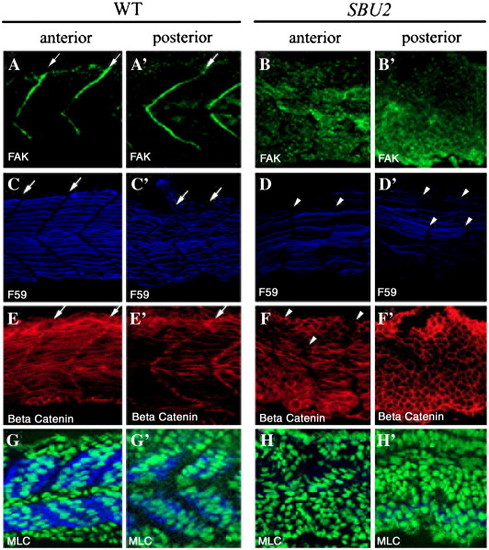
Somite boundary formation and muscle fiber differentiation are disrupted in SBU2 mutants. Confocal sections of anterior (A–H) and posterior (A′–H′) portions of the trunk region 29-somite stage embryos at approximately same medial–lateral and anterior–posterior locations; anterior to the left. Focal adhesion marker phosphorylated Fak is in green; slow muscle marker F59 is in blue; β-catenin which outlines the cell is in red. In wild-type embryos, phosphorylated Fak accumulates at myotome borders, revealing regularly formed somite boundaries (A and A′). On the other hand, there is no specific accumulation of phosphorylated FAK in SBU2 mutants (B and B′). The number of the slow muscle fibers is lower both in anterior and posterior (D and D′) compared to wild-type muscle fibers (C and C′). In wild-type embryos, fast muscle precursors elongate and attach to the anterior and posterior somite borders (E and E′). In SBU2 mutant embryos, some fast cells elongate in the anterior of the embryos (F). However, in the posterior, few, if any, fast cells elongate (F′). Likewise, the MLC antibody F310 staining (blue) is abundant in wild-type muscle (G and G′) but absent in SBU2 mutant embryos (H and H′), nuclei are labeled with cytoxgreen. Therefore, fast muscle fiber morphogenesis is severely disrupted in SBU2 mutant embryos. Arrows denote somite boundaries. Arrowheads denote improper and degenerated somite boundaries. EXPRESSION / LABELING:
|
|
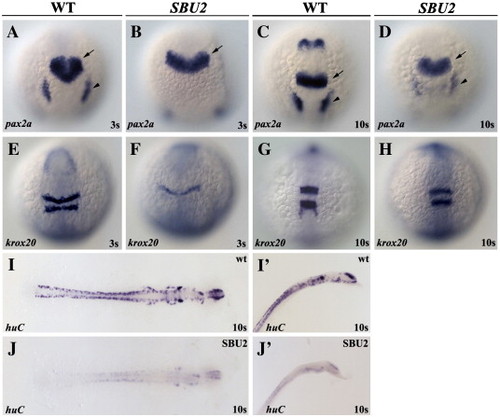
SBU2 mutants are not neurogenic. Whole mount RNA in situ hybridization of neural markers in wild-type and SBU2 mutants. (A–J) Dorsal views; (A–H) anterior to the top, (I, J) flat mounted, anterior to the right. Panels I′, J′ are lateral views of panels I and J, anterior to the top right. Morphology of the MHB domain expressing pax2a at early somitogenesis (B) indicates a delay in development of MHB in SBU2 mutants. Later, at the 10-somite stage (D) pax2a expression resembles much earlier stage wild-type expression domain (A). Likewise, although hindbrain marker krox20 expression is also delayed and relatively reduced (E and F) later in the development, expression pattern appears similar to wild-type embryos (G and H). RNA in situ hybridization using huC probe revealed that SBU2 embryos have reductions in early differentiating neurons (I, I′ and J, J′). Arrows denote MHB and arrowheads denote otic placode. EXPRESSION / LABELING:
|
|
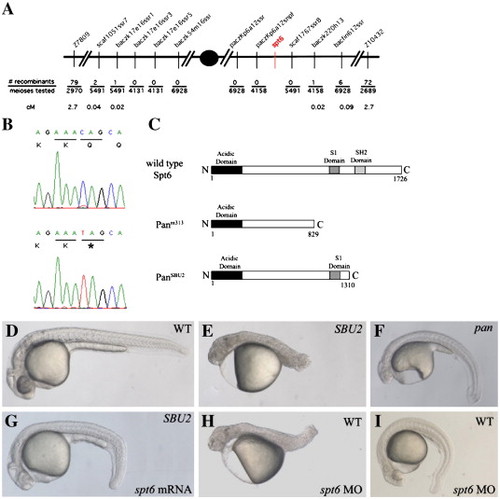
SBU2 phenotype is the result of a non-sense mutation on spt6 gene. SBU2 maps to linkage group 21 between Z7809 and Z10432 (A). Sequencing analysis revealed a C to T transition at the 3931st nucleotide of the coding sequence of the spt6 gene (B). Predicted Spt6 protein structure in wild-type, panm313 and panSBU2 mutant embryos (C). The premature stop codon in SBU2 is predicted to produce a truncated protein which lacks SH2 domain (C). The defects in homozygous SBU2 embryos can be rescued by injection of spt6 mRNA (G). Microinjection of spt6 morpholino into the progeny from intercrosses of wild-type siblings of SBU2 heterozygotes, phenocopies SBU2 mutants (H). However, in some wild-type stocks, the phenotype upon injection of spt6 morpholino is much weaker (I) and resembles pan phenotype (F). Panels D, E, G, and H are 27-h-old embryos; panels F and I are 48-h-old embryos. Asterisk denotes stop codon in panel B. |
|
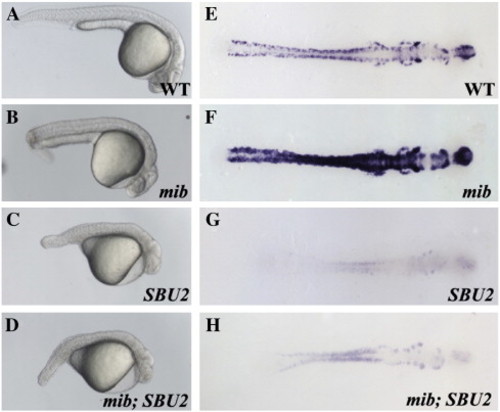
mib;SBU2 double mutants reveal an epistatic interaction between Spt6 and Notch pathway. (A–D) Lateral views of 27-h-old embryos, anterior to the right. (E–H) Dorsal views of huC stained embryos, anterior to the right. All embryos were genotyped following photography. The phenotype of mib;SBU2 mutants (D) resembles SBU2 single mutants (C), rather than mib single mutant embryos (B). However, huC expression profile in mib;SBU2 double mutants (H) is stronger than SBU2 mutants (G) but not as strong as either wild-type (E) or mib single mutants (F). EXPRESSION / LABELING:
|

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 307(2), Kok, F.O., Oster, E., Mentzer, L., Hsieh, J.C., Henry, C.A., and Sirotkin, H.I., The role of the SPT6 chromatin remodeling factor in zebrafish embryogenesis, 214-226, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.